Minireview: The neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals - PubMed (original) (raw)
Review
. 2007 Dec;148(12):5640-7.
doi: 10.1210/en.2007-1083. Epub 2007 Sep 27.
Affiliations
- PMID: 17901227
- PMCID: PMC3423957
- DOI: 10.1210/en.2007-1083
Review
Minireview: The neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals
Ilia N Karatsoreos et al. Endocrinology. 2007 Dec.
Abstract
Circadian rhythms in physiology and behavior are regulated by a master clock resident in the suprachiasmatic nucleus (SCN) of the hypothalamus, and dysfunctions in the circadian system can lead to serious health effects. This paper reviews the organization of the SCN as the brain clock, how it regulates gonadal hormone secretion, and how androgens modulate aspects of circadian behavior known to be regulated by the SCN. We show that androgen receptors are restricted to a core SCN region that receives photic input as well as afferents from arousal systems in the brain. We suggest that androgens modulate circadian behavior directly via actions on the SCN and that both androgens and estrogens modulate circadian rhythms through an indirect route, by affecting overall activity and arousal levels. Thus, this system has multiple levels of regulation; the SCN regulates circadian rhythms in gonadal hormone secretion, and hormones feed back to influence SCN functions.
Figures
Fig. 1
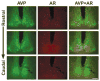
Photomicrographs depict double-label immunocytochemistry for AVP (green) and AR (red) from the rostral to caudal SCN in a male mouse. AVP delineates the shell region of the SCN, whereas AR is largely restricted to the core region. Scale bar, 150 _μ_m.
Fig. 2
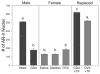
Average number of AR-positive cell nuclei within the SCN of male and female mice under various hormonal conditions. AR levels are high in intact mice, but reduced after castration (GDX). In contrast, AR levels are low in females and not affected by either the estrous cycle (D, diestrus; E, estrus) or by ovariectomy (OVX). Replacing castrated males with a sc testosterone propionate (TP) capsule for 7d restores AR levels, whereas similar treatment of OVX females also results in increased levels, although not to the same levels as TP-replaced males. n = 4–6 animals per group. Columns sharing a common letter are not statistically different from each other, P < 0.01. Modified from Iwahana et al. (116).
Fig. 3
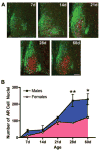
A, Photomicrographs depict AR staining in the mid-SCN of male mice at 7, 14, 21, 28, and 60 d of age. AVP is labeled in green, and AR nuclei are labeled in red. Scale bar, 100 _μ_m. B, AR staining in the mid-SCN of females (pink, ○) and males (blue, ●) during postnatal development. *, _P <_0.01; **, _P <_0.001. n = 3–4 animals per group.
Fig. 4
Schematic representation of differences between the site of action of estrogenic and androgenic hormones on the SCN and its inputs. Upper panel shows ER-rich nuclei (gray) that project to the SCN, including the retina, the intergeniculate leaflet, and the dorsal raphe nucleus, via the median raphe. Lower panel shows AR-rich areas in the core SCN and also the dorsal raphe. Testosterone can be aromatized into estradiol and thus may have dual androgenic/estrogenic impacts on the system. The SCN regulates circadian timing in physiology and behavior by sending outputs to autonomic and neuroendocrine systems. The SCN core receives direct input from the retina, the intergeniculate leaflet, and the median raphe nucleus, providing resetting information from photic cues and from activity/exercise.
References
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: the mind’s clock. New York: Oxford University Press; 1991.
- Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13:100–112. - PubMed
- Gillette MU, Reppert SM. The hypothalamic suprachiasmatic nuclei: circadian patterns of vasopressin secretion and neuronal activity in vitro. Brain Res Bull. 1987;19:135–139. - PubMed