Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4 - PubMed (original) (raw)
. 2007 Sep 28;317(5846):1930-4.
doi: 10.1126/science.1145373.
Son N Lam, Priyamvada Acharya, Min Tang, Shi-Hua Xiang, Syed Shahzad-Ul Hussan, Robyn L Stanfield, James Robinson, Joseph Sodroski, Ian A Wilson, Richard Wyatt, Carole A Bewley, Peter D Kwong
Affiliations
- PMID: 17901336
- PMCID: PMC2278242
- DOI: 10.1126/science.1145373
Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4
Chih-Chin Huang et al. Science. 2007.
Abstract
The CCR5 co-receptor binds to the HIV-1 gp120 envelope glycoprotein and facilitates HIV-1 entry into cells. Its N terminus is tyrosine-sulfated, as are many antibodies that react with the co-receptor binding site on gp120. We applied nuclear magnetic resonance and crystallographic techniques to analyze the structure of the CCR5 N terminus and that of the tyrosine-sulfated antibody 412d in complex with gp120 and CD4. The conformations of tyrosine-sulfated regions of CCR5 (alpha-helix) and 412d (extended loop) are surprisingly different. Nonetheless, a critical sulfotyrosine on CCR5 and on 412d induces similar structural rearrangements in gp120. These results now provide a framework for understanding HIV-1 interactions with the CCR5 N terminus during viral entry and define a conserved site on gp120, whose recognition of sulfotyrosine engenders posttranslational mimicry by the immune system.
Figures
Fig. 1
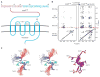
Structure of the tyrosine-sulfated N terminus of CCR5 in the gp120-bound conformation. (A) CCR5 sequence and schematic of its insertion in the cell membrane. Sequence letters in purple correspond to residues in CCR52-15, with sulfotyrosines (Tys) critical for interaction with HIV-1 highlighted in black. ECLs are labeled, and disulfide bridges (-SS-) depicted. (B) 2D NOESY spectra for CCR52-15 free in solution (left) and in the presence of gp120–CD4 (right). NMR samples (20 mM phosphate, 50 mM NaCl, pH 6.85) contained 800 μM CCR52-15 in the presence of 20 μM gp120-CD4 and were recorded at 500 MHz, 300 K, mixing time = 150 msec. Sequential NH(i)–CαH(_i_-1) NOEs were observed between every residue, thereby confirming sequential assignments, and predicted intraresidue NOEs were observed for all residues. No correlations beyond sequential NOEs were observed between residues 2 and 7, indicating that this region of CCR5 was extended or disordered. In contrast, NOEs from CαH(i) to NH(i + 1,2,3) and from NH(i) to NH(i + 1,2,3) were observed for residues 9 to 15 (fig. S1), indicating an ordered α-helical structure (33). (C) Structure of the ordered region of gp120-bound CCR52-15. Stereoview (left) of 25 lowest energy-simulated annealing structures superimposed by fitting to the backbone of residues 9 to 15. Structural statistics are provided in table S2. Backbone appears in blue, amide hydrogens (9 to 15) in blue, side chains (11 to 13) in green, and Tys 10 and Tys 14 in red. Ribbon diagram (right) of restrained minimized mean structure with side chains in stick representations.
Fig. 2
Structure of the tyrosine-sulfated antibody 412d in complex with HIV-1 gp120 and CD4. (A) Ribbon representation. CD4 is yellow, the heavy chain of Fab 412d is dark blue, the light chain is cyan, and gp120 is gray, except for the V3 loop, which is orange. The CDR H3 loop of 412d is red, with sulfotyrosines depicted in stick representation. (B) Close-up, with molecular surface of gp120 in gray and sulfotyrosines of 412d (red labels) and select residues of gp120 (black labels) in stick representation. Dotted lines represent coordinating hydrogen bonds between gp120 and the sulfate group of Tys100c412d. The sulfate of Tys 100c412d makes a full complement of ionic interactions: a salt bridge to Arg 298gp120 and hydrogen bonds to the side-chain nitrogen of Asn 302gp120, the side-chain hydroxyl of Thr 303gp120, and the main-chain amides of 302gp120, 303gp120, and 441gp120 (34).
Fig. 3
Interaction of the N terminus of CCR5 with HIV-1 gp120-CD4. (A) Molecular docking. The 20 lowest energy structures (black) from 200 docking runs of CCR52-15 are shown in stick representation. Despite initial random orientations, all favorable docking solutions had Tys 14 binding at the bridging sheet-V3 interface; none had Tys 10 at this cleft. Ribbon representations illustrate CD4 in yellow, gp120 in gray (with V3 in orange), and the lowest energy structure of CCR57-15 in purple. (B) Close-up, with molecular surface of gp120 in gray and select residues of gp120 (black labels) and CCR5 (purple labels) in stick representation. (C) Saturation transfer difference NMR spectrum of CCR52-15 in the presence of gp120–CD4 (red) overlaid on a control 1H spectrum (black). Experimental conditions were identical to those used for NOE experiments, except that the carrier was set at –1 and 50 parts per million for on- and off-resonance saturation, respectively. The intensities of the most strongly enhanced peaks (Tys 14 and Tyr 15) have been normalized to the corresponding signals in the control spectrum. Peak assignments made by 2D NMR (table S1) appear above their corresponding doublet signals. Tys 14 and Tyr 15 show strong saturation transfer difference effects, whereas Tys 10 shows a medium effect and Tyr 3 a very weak effect. These effects correlate directly with the buried surface area of each tyrosine ring in the docked structure. See fig. S9 for overlaid spectra employing 1 to 7 s saturation. (D) Effect of CCR52-15 on the proteolytic sensitivity of the V3. Electrophoresis on an 8 to 25% gradient SDS polyacrylamide gel shows the results of thrombin digestion on gp120 (core with V3; YU2 R5 strain of HIV-1) alone, or in the presence of sCD4 or sCD4 and CCR52-15 (35). (E) Structural intermediates of HIV-1 entry. At far left, a single monomer of unliganded gp120 (gray) is shown with separated β-hairpins. The threefold axis, from which gp41 interacts in the functional oligomer, is labeled with the number 3. In the CD4-bound state, the bridging sheet assembles, and the V3 (orange) is exposed and flexible. The next state involves either (upper pathway) the interaction of the CCR5-ECL2 region with the V3 tip or (lower pathway) the interaction of the CCR5 N terminus, which induces rigidification of the V3 stem. Engagement of CCR5 at both N terminus and ECL2 region triggers additional conformational changes leading to HIV-1 entry.
Fig. 4
A conserved site for binding sulfotyrosine on HIV-1 gp120. (A) Alterations of the V3 base to accommodate binding of sulfotyrosine. The gp120 (gray) region around the V3 loop (orange) is illustrated in ribbon diagram, with an overlying semitrans-parent surface for unbound (left panel) and bound (right panel) conformations. Binding of the CCR5 N terminus (purple) or the 412d CDR H3 (red), each with two sulfotyrosines (stick representation, with red and purple labels), alters the V3 base, forming a sulfotyrosine binding pocket and a rigid β-hairpin. (B) Close-up of the conserved sulfotyrosine binding pocket. The orientation shown is similar to that in Figs. 2B and 3B [~90° from (A) about a diagonal axis, as defined by the long axis of the V3 from (A)]. Tys 14CCR5 is shown in purple, with Tys 100c412d in red. Select residues of gp120 are shown in stick representation and labeled in black. Hydrogen bonds coordinating the buried sulfate groups in each are depicted with dotted lines.
Similar articles
- Tyrosine-sulfated V2 peptides inhibit HIV-1 infection via coreceptor mimicry.
Cimbro R, Peterson FC, Liu Q, Guzzo C, Zhang P, Miao H, Van Ryk D, Ambroggio X, Hurt DE, De Gioia L, Volkman BF, Dolan MA, Lusso P. Cimbro R, et al. EBioMedicine. 2016 Aug;10:45-54. doi: 10.1016/j.ebiom.2016.06.037. Epub 2016 Jun 26. EBioMedicine. 2016. PMID: 27389109 Free PMC article. - Tyrosine-sulfate isosteres of CCR5 N-terminus as tools for studying HIV-1 entry.
Lam SN, Acharya P, Wyatt R, Kwong PD, Bewley CA. Lam SN, et al. Bioorg Med Chem. 2008 Dec 1;16(23):10113-20. doi: 10.1016/j.bmc.2008.10.005. Epub 2008 Oct 5. Bioorg Med Chem. 2008. PMID: 18952441 Free PMC article. - Binding thermodynamics of the N-terminal peptide of the CCR5 coreceptor to HIV-1 envelope glycoprotein gp120.
Brower ET, Schön A, Klein JC, Freire E. Brower ET, et al. Biochemistry. 2009 Feb 3;48(4):779-85. doi: 10.1021/bi8021476. Biochemistry. 2009. PMID: 19170639 Free PMC article. - Tyrosine sulfation of human antibodies contributes to recognition of the CCR5 binding region of HIV-1 gp120.
Choe H, Li W, Wright PL, Vasilieva N, Venturi M, Huang CC, Grundner C, Dorfman T, Zwick MB, Wang L, Rosenberg ES, Kwong PD, Burton DR, Robinson JE, Sodroski JG, Farzan M. Choe H, et al. Cell. 2003 Jul 25;114(2):161-70. doi: 10.1016/s0092-8674(03)00508-7. Cell. 2003. PMID: 12887918 - CCR5 mimicry by sulfated human anti-HIV-1 antibodies.
Lin G, Hoxie JA. Lin G, et al. Cell. 2003 Jul 25;114(2):147-8. doi: 10.1016/s0092-8674(03)00564-6. Cell. 2003. PMID: 12887913
Cited by
- C-C Chemokine Receptor 7 Promotes T-Cell Acute Lymphoblastic Leukemia Invasion of the Central Nervous System via β2-Integrins.
Cardona CI, Rodriguez A, Torres VC, Sanchez A, Torres A, Vazquez AE, Wagler AE, Brissette MA, Bill CA, Vines CM. Cardona CI, et al. Int J Mol Sci. 2024 Sep 6;25(17):9649. doi: 10.3390/ijms25179649. Int J Mol Sci. 2024. PMID: 39273598 Free PMC article. - Dimethyl sulfate and diisopropyl sulfate as practical and versatile O-sulfation reagents.
Yue S, Ding G, Zheng Y, Song C, Xu P, Yu B, Li J. Yue S, et al. Nat Commun. 2024 Feb 29;15(1):1861. doi: 10.1038/s41467-024-46214-x. Nat Commun. 2024. PMID: 38424087 Free PMC article. - Tyrosine Sulfation at Antibody Light Chain CDR-1 Increases Binding Affinity and Neutralization Potency to Interleukine-4.
D'Antona AM, Lee JM, Zhang M, Friedman C, He T, Mosyak L, Bennett E, Lin L, Silverman M, Cometa F, Meade C, Hageman T, Sousa E, Cohen J, Marquette K, Ferguson D, Zhong X. D'Antona AM, et al. Int J Mol Sci. 2024 Feb 5;25(3):1931. doi: 10.3390/ijms25031931. Int J Mol Sci. 2024. PMID: 38339208 Free PMC article. - The chemokine receptor CCR5: multi-faceted hook for HIV-1.
Faivre N, Verollet C, Dumas F. Faivre N, et al. Retrovirology. 2024 Jan 23;21(1):2. doi: 10.1186/s12977-024-00634-1. Retrovirology. 2024. PMID: 38263120 Free PMC article. Review. - Editorial: Mechanisms and strategies of unconventional antibody diversification for greater immune adaptability.
Dimitrov JD, Mwangi W, Zhong X. Dimitrov JD, et al. Front Immunol. 2023 Aug 31;14:1267556. doi: 10.3389/fimmu.2023.1267556. eCollection 2023. Front Immunol. 2023. PMID: 37727783 Free PMC article. No abstract available.
References
- Wyatt R, Sodroski J. Science. 1998;280:1884. - PubMed
- Berger EA, Murphy PM, Farber JM. Annu Rev Immunol. 1999;17:657. - PubMed
- Palczewski K, et al. Science. 2000;289:739. - PubMed
- Liu R, et al. Cell. 1996;86:367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI060354/AI/NIAID NIH HHS/United States
- U19 AI067854/AI/NIAID NIH HHS/United States
- U19 AI067854-03/AI/NIAID NIH HHS/United States
- Z99 AI999999/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials