Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis - PubMed (original) (raw)
Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis
Lars Johan Nissen et al. J Clin Invest. 2007 Oct.
Abstract
Tumors produce multiple growth factors, but little is known about the interplay between various angiogenic factors in promoting tumor angiogenesis, growth, and metastasis. Here we show that 2 angiogenic factors frequently upregulated in tumors, PDGF-BB and FGF2, synergistically promote tumor angiogenesis and pulmonary metastasis. Simultaneous overexpression of PDGF-BB and FGF2 in murine fibrosarcomas led to the formation of high-density primitive vascular plexuses, which were poorly coated with pericytes and VSMCs. Surprisingly, overexpression of PDGF-BB alone in tumor cells resulted in dissociation of VSMCs from tumor vessels and decreased recruitment of pericytes. In the absence of FGF2, capillary ECs lacked response to PDGF-BB. However, FGF2 triggers PDGFR-alpha and -beta expression at the transcriptional level in ECs, which acquire hyperresponsiveness to PDGF-BB. Similarly, PDGF-BB-treated VSMCs become responsive to FGF2 stimulation via upregulation of FGF receptor 1 (FGFR1) promoter activity. These findings demonstrate that PDGF-BB and FGF2 reciprocally increase their EC and mural cell responses, leading to disorganized neovascularization and metastasis. Our data suggest that intervention of this non-VEGF reciprocal interaction loop for the tumor vasculature could be an important therapeutic target for the treatment of cancer and metastasis.
Figures
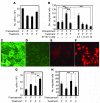
Figure 1. Reciprocal regulation of proliferation and migration of ECs and VSMCs.
(A) BCE cell proliferation. BCE cells were pretreated with FGF2 (F) or PDGF-BB (P), followed by stimulation with either FGF2 or PDGF-BB. Cell proliferation was measured 72 hours after treatment by counting cell numbers. (B) Migration of FGF2- or PDGF-BB–pretreated BCE cells were assayed in Boyden chambers for 4 hours in the presence and absence of STI571. Migrating cells were counted under a light microscope. (C–F) Rat VSMCs (C) and BCE cells (E) were immunostained with an anti–α-SMA antibody. Uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocynaine perchlorate–labeled Ac-LDL by VSMCs (D) and BCE cells (F) was analyzed. (G and H) VSMC proliferation (G) and migration (H) stimulated by FGF2 and/or PDGF-BB. The data represent means of average determinants ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bar: 50 μm.
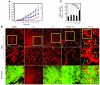
Figure 2. Angiogenic synergism.
PBS (A), PDGF-BB (B and E), FGF2 (C and F), or PDGF-BB plus FGF2 (D and G) together with a slow-release polymer was implanted into the mouse cornea, and corneal neovascularization was photographed on day 5 after implantation. (E–K) Growth factor–implanted corneas were stained with an anti-CD31 antibody, and corneal neovascularization was quantified by measuring vessel areas from 10–12 animals. Arrows point to CD31-positive microvessels. The data represent means of average determinants ± SEM. ***P < 0.001. Scale bar: 100 μm (A–G); 25 μm (H–J).
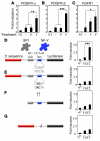
Figure 3. Regulation of PDGFR and FGFR promoter activity.
PDGFR-α (A) and PDGFR-β (B) promoters were fused with luciferase gene as a reporter system to detect promoter activity in ECs. After transfection, BCE cells were incubated with PDGF-BB or FGF2 for 40 hours, and luciferase activity was determined. NT, nontransfected. Dash indicates PBS treated. (C) Rat VSMCs were transfected with FGFR1-CAT construct and FGFR1 promoter activity was determined by measuring the CAT activity. (D–G) The intact (D) and various mutated (E–G) PDGFR-β promoters were fused with luciferase reporter gene, and promoter activity was measured in growth factor–stimulated BCE cells. A β-galactosidase construct was used as a control to standardize the transfection system in all experiments. The data represent means of average determinants ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 4. Regulation of mRNA and protein expression of PDGFRs and signaling pathways.
(A) RT-PCR was used to quantitatively measure PDGFR-α and PDGFR-β expression levels in FGF2- or PDGF-BB–treated BCE cells for the indicated time points. GAPDH was used as a standard control. (B) BCE cell lysates treated with different concentrations of FGF2 were immunoblotted with an anti–PDGFR-β antibody and an anti–p-PDGFR-β antibody. An anti–β-actin antibody was used as a standard control for loading levels. (C–J) In situ hybridization. PBS- (C and G), PDGF-BB– (D and H), FGF2- (E and I), or PDGF-BB/FGF2–implanted (F and J) corneas were hybridized in situ with probes for PDGFR-α (C–F) and PDGFR-β (G–J) at day 5 after growth factor implantation. Arrows point to positive signals. (K) Analysis of signaling components. FGF2-pretreated BCE cells were treated with PDGF-BB for indicated time points, and cell lysates were immunoblotted with different antibodies. β-Actin was immunoblotted as a control for loading levels. Scale bar: 10 μm.
Figure 5. Tumor growth rates and vasculature.
(A) Growth factor– or vector-transfected tumor cells were subcutaneously implanted in SCID mice, and tumor growth was measured daily. Green line, FGF2 + PDGF-BB; red line, FGF2; blue line, vector + FGF2; purple line, vector and black PDGF-BB. (B) At day 13 after tumor cell implantation, tumors were removed and stained with an anti-CD31 antibody, and tumor blood vessels were analyzed by confocal microscopy using 3D projections. GFP-expressing tumor cells are green in color, and tumor blood vessels are presented in red. Arrows point to tip-cell sprouts from the vascular plexuses induced by FGF2 and PDGF-BB. (C) Quantification of CD31-positive tumor vessels from 8–12 randomized cryosectioned fields. The data represent means of average determinants ± SEM. *P < 0.05; ***P < 0.001. Scale bar: 100 μm (B, upper and lower panels); 50 μm (B, middle panels). V, vector.
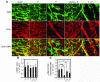
Figure 6. Interaction between VSMCs and ECs in tumors.
At day 14 after implantation, tumor tissues were double-stained with an anti-CD31 antibody and an anti–α-SMA antibody. (A) The CD31- (red) and α-SMA–positive (green) signals were revealed by Alexa Fluor 555– and Alexa Fluor 647–labeled antibodies, respectively, using single-layer projections in a confocal microscope. Overlapping double-positive signals are in yellow color. (B and C) Total numbers of α-SMA–positive vessels were randomly counted from 12 fields/group, and percentages of α-SMA–positive vessels versus total CD31-positive vessels were calculated. The data represent means of average determinants ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bar: 50 μm.
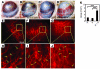
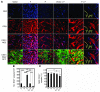
Figure 7. Interaction between pericytes and ECs in tumors.
At day 10 after implantation, tumor tissues were double-stained with an anti-CD31 antibody and an anti-NG2 antibody. (A) The CD31- (red) and NG2-positive (blue) signals were revealed by Alexa Fluor 555– and Cy5-labeled antibodies, respectively, using single-layer projections in a confocal microscope. Tumor cells were GFP positive (green). T, Intratumoral area; PT, peritumoral area. (B) Total numbers of NG2 positive vessels were randomly counted from 9 fields/group, and (C) percentages of NG2-positive vessels relative to total CD31-positive vessels were calculated. The data represent means of average determinants ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bar: 50 μm.
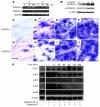
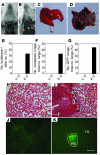
Figure 8. Stimulation of metastasis by coexpression of FGF2 and PDGF-BB in tumors.
Subcutaneous tumors were removed when they reached 1.5 cm3. (A and B) Mice were followed for 4 weeks, and pulmonary metastases of vector/FGF2 tumor–bearing (A) and PDGF-BB/FGF2 tumor–bearing (B) mice were examined by bioluminescence. Arrows point to luciferase-positive lung metastases. (E) Numbers of pulmonary luciferase–positive animals relative to total numbers of animals are presented as percentages. (C and D) Examples of lung morphology of vector/FGF2 tumor–bearing (C) and PDGF-BB/FGF2 tumor–bearing (D) mice and arrows in D panel point to visible lung metastases in the PDGF-BB/FGF2 group. (F) Numbers of lungs with visible pulmonary metastases versus total numbers of lungs are presented as percentages. (H and I) Lung tissues were stained with H&E and metastatic nodules were validated in the PDGF-BB/FGF2 group (I). (H) No metastasis was visible in the vector/FGF2 tumor-bearing control group. (J and K) GFP-positive metastases were revealed by analysis of lung sections under a fluorescent microscope of vector/FGF2- (J) and PDGF-BB/FGF2-tumor bearing (K) mice. (G) Numbers of lungs with GFP-positive metastases relative to total numbers of lungs are presented as percentages. Met, metastasis; HL, healthy lung tissue. Scale bars: 100 μm.
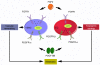
Figure 9. Schematic representation of reciprocal interplay between FGF2 and PDGF-BB in promoting tumor angiogenesis and metastasis.
FGF2 induces expression of PDGFR-α and -β in ECs, which are activated by PDGF-BB. Activation of PDGFRs leads to enhanced EC migration, which would synergistically coordinate with FGF2-induced EC proliferation. Both proliferation and migration of ECs are essential steps of tumor angiogenesis and are manifested in vivo as angiogenic synergism, which promotes metastasis. Conversely, PDGF-BB upregulates FGFR1 expression in VSMCs, which mediates proliferation signals triggered by FGF2. The FGF2-induced VSMC proliferation synergistically cooperates with PDGF-BB–induced VSMC proliferation and migration to crosstalk to nascent vasculature for remodeling. The coordination between synergistic angiogenesis and synergistic vascular remodeling in facilitating a functional vasculature remains unknown. In the tumor environment, it appears that FGF2/PDGF-BB–induced synergistic angiogenesis is not well coordinated with the angiogenic effects of FGF2 and PDGF-BB vascular remodeling. As a consequence, these 2 factors induce disorganized vasculatures and promote cancer metastasis.
Comment in
- Why targeted therapy hasn't worked in advanced cancer.
Arbiser JL. Arbiser JL. J Clin Invest. 2007 Oct;117(10):2762-5. doi: 10.1172/JCI33190. J Clin Invest. 2007. PMID: 17909624 Free PMC article.
Similar articles
- R Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways.
Cao Y, Cao R, Hedlund EM. Cao Y, et al. J Mol Med (Berl). 2008 Jul;86(7):785-9. doi: 10.1007/s00109-008-0337-z. Epub 2008 Apr 8. J Mol Med (Berl). 2008. PMID: 18392794 Review. - VEGF-C regulates lymphangiogenesis and capillary stability by regulation of PDGF-B.
Onimaru M, Yonemitsu Y, Fujii T, Tanii M, Nakano T, Nakagawa K, Kohno R, Hasegawa M, Nishikawa S, Sueishi K. Onimaru M, et al. Am J Physiol Heart Circ Physiol. 2009 Nov;297(5):H1685-96. doi: 10.1152/ajpheart.00015.2009. Epub 2009 Sep 4. Am J Physiol Heart Circ Physiol. 2009. PMID: 19734356 - Overexpression of PDGF-BB decreases colorectal and pancreatic cancer growth by increasing tumor pericyte content.
McCarty MF, Somcio RJ, Stoeltzing O, Wey J, Fan F, Liu W, Bucana C, Ellis LM. McCarty MF, et al. J Clin Invest. 2007 Aug;117(8):2114-22. doi: 10.1172/JCI31334. J Clin Invest. 2007. PMID: 17641778 Free PMC article. - VEGF-A/VEGFR-2 and FGF-2/FGFR-1 but not PDGF-BB/PDGFR-β play important roles in promoting immature and inflammatory intraplaque angiogenesis.
Mao Y, Liu X, Song Y, Zhai C, Zhang L. Mao Y, et al. PLoS One. 2018 Aug 20;13(8):e0201395. doi: 10.1371/journal.pone.0201395. eCollection 2018. PLoS One. 2018. PMID: 30125282 Free PMC article. - Role of Pericytes in Diabetic Angiogenesis.
Wang T, Zang G, Zhang L, Sun Z, Liu J, Hou L, Wang Z. Wang T, et al. J Cardiovasc Pharmacol. 2022 Jan 1;79(1):e1-e10. doi: 10.1097/FJC.0000000000001147. J Cardiovasc Pharmacol. 2022. PMID: 34654782 Review.
Cited by
- Small Molecular-Sized Artesunate Attenuates Ocular Neovascularization via VEGFR2, PKCα, and PDGFR Targets.
Zong Y, Yuan Y, Qian X, Huang Z, Yang W, Lin L, Zheng Q, Li Y, He H, Gao Q. Zong Y, et al. Sci Rep. 2016 Aug 2;6:30843. doi: 10.1038/srep30843. Sci Rep. 2016. PMID: 27480521 Free PMC article. - Integrated omics characterization reveals reduced cancer indicators and elevated inflammatory factors after thermal ablation in non-small cell lung cancer patients.
Zhang X, Shao S, Song N, Yang B, Liu F, Tong Z, Wang F, Li J. Zhang X, et al. Respir Res. 2024 Aug 14;25(1):309. doi: 10.1186/s12931-024-02917-9. Respir Res. 2024. PMID: 39143582 Free PMC article. - PC-3 prostate carcinoma cells release signal substances that influence the migratory activity of cells in the tumor's microenvironment.
Voss MJ, Niggemann B, Zänker KS, Entschladen F. Voss MJ, et al. Cell Commun Signal. 2010 Jul 13;8:17. doi: 10.1186/1478-811X-8-17. Cell Commun Signal. 2010. PMID: 20626867 Free PMC article. - Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis.
Heishi T, Hosaka T, Suzuki Y, Miyashita H, Oike Y, Takahashi T, Nakamura T, Arioka S, Mitsuda Y, Takakura T, Hojo K, Matsumoto M, Yamauchi C, Ohta H, Sonoda H, Sato Y. Heishi T, et al. Am J Pathol. 2010 Apr;176(4):1950-8. doi: 10.2353/ajpath.2010.090829. Epub 2010 Feb 4. Am J Pathol. 2010. PMID: 20133819 Free PMC article. - Lenvatinib for effectively treating antiangiogenic drug-resistant nasopharyngeal carcinoma.
Sun Q, Wang Y, Ji H, Sun X, Xie S, Chen L, Li S, Zeng W, Chen R, Tang Q, Zuo J, Hou L, Hosaka K, Lu Y, Liu Y, Ye Y, Yang Y. Sun Q, et al. Cell Death Dis. 2022 Aug 19;13(8):724. doi: 10.1038/s41419-022-05171-3. Cell Death Dis. 2022. PMID: 35985991 Free PMC article.
References
- Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. - PubMed
- Ellis L.M., Fidler I.J. Angiogenesis and metastasis. Eur. J. Cancer. 1996;32A:2451–2460. - PubMed
- Ferrara N., Kerbel R.S. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. - PubMed
- Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. - PubMed
- Hanahan D., Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous