Sticky overhangs enhance siRNA-mediated gene silencing - PubMed (original) (raw)
Sticky overhangs enhance siRNA-mediated gene silencing
Anne-Laure Bolcato-Bellemin et al. Proc Natl Acad Sci U S A. 2007.
Abstract
siRNA delivery to cells offers a convenient and powerful means of gene silencing that bypasses several barriers met by gene delivery. However, nonviral vectors, and especially polymers, form looser complexes with siRNA than with plasmid DNA. As a consequence, exchange of siRNA for larger polymeric anions such as proteoglycans found outside cells and at their surface may occur and lower delivery. We show here that making siRNAs "gene-like," via short complementary A(5-8)/T(5-8) 3' overhangs, increases complex stability, and hence RNase protection and gene silencing in vitro up to 10-fold. After decomplexation in the cytoplasm, sticky siRNA (ssiRNA) concatemers fall apart. ssiRNAs are therefore not inducing antiviral responses, as shown by the absence of IFN-beta production. Finally, transfection experiments in the mouse lung show that ssiRNA should be particularly suited to silencing with linear polyethylenimine in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Confocal microscopy sections of HeLa cells transfected with a fluorescent siRNA complexed to a cationic lipid or a cationic polymer. Lipid complexes are found in the perinuclear lysosomal compartment (Upper, arrowheads). Polymer complexes are mostly found at the cell surface (Lower, arrowheads) and some times inside the cell (arrows).
Fig. 2.
Gel electrophoresis of siRNA/PEI complexes incubated with increasing amounts of HS. Because of aggregation, siRNA/PEI complexes remain in the well (0 eq HS). Incubation for 30 min with 20–40 (wt/wt) eq HS releases siRNA (Left and Right), but not ssiRNA (Center), from the complexes.
Fig. 3.
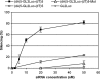
Endogeneous luciferase silencing with ssiRNA/PEI complexes requires 10-fold less RNA. A549Luc cells stably expressing GL3 luciferase were incubated with increasing concentrations of ssiRNAs complexed to PEI (N/P = 5). Control RNAs were the corresponding nonsticky siRNA of similar length and the classical GL3 siRNA with 3′(dT)2 overhangs. Luciferase inhibition was measured after 48 h.
Fig. 4.
ssiRNA-mediated silencing is sequence-specific. Sequences with three mismatches (○ and □) or with a single mismatch (■) in their GL3Luc sequence do not decrease endogeneous GL3 luciferase expression. Experimental conditions are as in Fig. 3.
Fig. 5.
Hybridization of identical (dT)n overhangs with a complementary oligodeoxyadenylate significantly enhances silencing. Experimental conditions are as in Fig. 3.
Fig. 6.
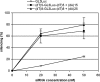
siRNA oligomerization enhances silencing when delivered by the cationic lipid formulation jetSi-ENDO (N/P = 6). Hybridization conditions are as in Fig. 5, and other experimental conditions are as in Fig. 3.
Fig. 7.
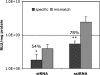
Luciferase gene silencing in the mouse lung. OF1 mice were coinjected retroorbitally with a luciferase-bearing plasmid and siRNA complexed separately with jetPEI. Luciferase gene expression was measured after 24 h. ssiRNA showed significantly higher gene silencing than classical siRNA (∗, P < 0.05; ∗∗, P < 0.01).
Similar articles
- Effect of siRNA nuclease stability on the in vitro and in vivo kinetics of siRNA-mediated gene silencing.
Bartlett DW, Davis ME. Bartlett DW, et al. Biotechnol Bioeng. 2007 Jul 1;97(4):909-21. doi: 10.1002/bit.21285. Biotechnol Bioeng. 2007. PMID: 17154307 - Rational design and in vitro and in vivo delivery of Dicer substrate siRNA.
Amarzguioui M, Lundberg P, Cantin E, Hagstrom J, Behlke MA, Rossi JJ. Amarzguioui M, et al. Nat Protoc. 2006;1(2):508-17. doi: 10.1038/nprot.2006.72. Nat Protoc. 2006. PMID: 17406276 - Synthesis of poly(propylene glycol)-block-polyethylenimine triblock copolymers for the delivery of nucleic acids.
Brissault B, Leborgne C, Scherman D, Guis C, Kichler A. Brissault B, et al. Macromol Biosci. 2011 May 12;11(5):652-61. doi: 10.1002/mabi.201000404. Epub 2011 Feb 8. Macromol Biosci. 2011. PMID: 21305695 - Gene silencing through RNA interference (RNAi) in vivo: strategies based on the direct application of siRNAs.
Aigner A. Aigner A. J Biotechnol. 2006 Jun 25;124(1):12-25. doi: 10.1016/j.jbiotec.2005.12.003. Epub 2006 Jan 18. J Biotechnol. 2006. PMID: 16413079 Review. - Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung.
Günther M, Lipka J, Malek A, Gutsch D, Kreyling W, Aigner A. Günther M, et al. Eur J Pharm Biopharm. 2011 Apr;77(3):438-49. doi: 10.1016/j.ejpb.2010.11.007. Epub 2010 Nov 18. Eur J Pharm Biopharm. 2011. PMID: 21093588 Review.
Cited by
- Preparation and Characterization of siRNA-Loaded Liposomes.
Liu Y, Huang L. Liu Y, et al. Methods Mol Biol. 2021;2282:159-169. doi: 10.1007/978-1-0716-1298-9_10. Methods Mol Biol. 2021. PMID: 33928575 - Performance of Pyridylthiourea-Polyethylenimine Polyplex for siRNA-Mediated Liver Cancer Therapy in Cell Monolayer, Spheroid, and Tumor Xenograft Models.
Gossart JB, Pascal E, Meyer F, Heuillard E, Gonçalves M, Gossé F, Robinet E, Frisch B, Seguin C, Zuber G. Gossart JB, et al. Glob Chall. 2017 May 19;1(4):1700013. doi: 10.1002/gch2.201700013. eCollection 2017 Jul 14. Glob Chall. 2017. PMID: 31565271 Free PMC article. - Tumor-targeted delivery of siRNA by non-viral vector: safe and effective cancer therapy.
Chen Y, Huang L. Chen Y, et al. Expert Opin Drug Deliv. 2008 Dec;5(12):1301-11. doi: 10.1517/17425240802568505. Expert Opin Drug Deliv. 2008. PMID: 19040393 Free PMC article. Review. - Antibody-mediated targeting of siRNA via the human insulin receptor using avidin-biotin technology.
Xia CF, Boado RJ, Pardridge WM. Xia CF, et al. Mol Pharm. 2009 May-Jun;6(3):747-51. doi: 10.1021/mp800194y. Mol Pharm. 2009. PMID: 19093871 Free PMC article. - RNA interference for glioblastoma therapy: Innovation ladder from the bench to clinical trials.
Lozada-Delgado EL, Grafals-Ruiz N, Vivas-Mejía PE. Lozada-Delgado EL, et al. Life Sci. 2017 Nov 1;188:26-36. doi: 10.1016/j.lfs.2017.08.027. Epub 2017 Aug 31. Life Sci. 2017. PMID: 28864225 Free PMC article. Review.
References
- Morris KV, Rossi JJ. Curr Opin Mol Ther. 2006;8:115–121. - PubMed
- Manoharan M. Curr Opin Chem Biol. 2004;8:570–579. - PubMed
- Aigner A. J Biotechnol. 2006;124:12–25. - PubMed
- Goula D, Remy JS, Erbacher P, Wasowicz M, Levi G, Abdallah B, Demeneix BA. Gene Ther. 1998;5:712–717. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous