Maternal immune activation alters fetal brain development through interleukin-6 - PubMed (original) (raw)
Maternal immune activation alters fetal brain development through interleukin-6
Stephen E P Smith et al. J Neurosci. 2007.
Abstract
Schizophrenia and autism are thought to result from the interaction between a susceptibility genotype and environmental risk factors. The offspring of women who experience infection while pregnant have an increased risk for these disorders. Maternal immune activation (MIA) in pregnant rodents produces offspring with abnormalities in behavior, histology, and gene expression that are reminiscent of schizophrenia and autism, making MIA a useful model of the disorders. However, the mechanism by which MIA causes long-term behavioral deficits in the offspring is unknown. Here we show that the cytokine interleukin-6 (IL-6) is critical for mediating the behavioral and transcriptional changes in the offspring. A single maternal injection of IL-6 on day 12.5 of mouse pregnancy causes prepulse inhibition (PPI) and latent inhibition (LI) deficits in the adult offspring. Moreover, coadministration of an anti-IL-6 antibody in the poly(I:C) model of MIA prevents the PPI, LI, and exploratory and social deficits caused by poly(I:C) and normalizes the associated changes in gene expression in the brains of adult offspring. Finally, MIA in IL-6 knock-out mice does not result in several of the behavioral changes seen in the offspring of wild-type mice after MIA. The identification of IL-6 as a key intermediary should aid in the molecular dissection of the pathways whereby MIA alters fetal brain development, which can shed new light on the pathophysiological mechanisms that predispose to schizophrenia and autism.
Figures
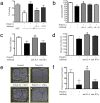
Figure 1.
The offspring of mice given injections of recombinant IL-6 exhibit abnormal behaviors. a, Offspring of mice given injections of IL-6 but not IFNγ have a PPI deficit at a prepulse intensity of 85 dB (F(2,79) = 4.369; p < 0.05). *p < 0.05 versus control. b, PE offspring of control mice show normal LI compared with NPE animals, as do PE offspring of IFNγ-injected mothers. Offspring of IL-6-injected mothers, in contrast, do not demonstrate significant LI (F(3,103) = 10.22; p < 0.0001). *p < 0.05 versus NPE; **p < 0.001 versus NPE.
Figure 2.
Abnormal behavior in MIA offspring is prevented by maternal treatment with anti-IL-6 antibody. a, Offspring of mice treated with poly(I:C) lack LI. Coinjection of anti-IL-6 with poly(I:C) restores significant LI, whereas coinjection of anti-IFNγ does not (F(4,132) = 7.566; p < 0.0001). **p < 0.001 versus NPE; *p < 0.01 versus NPE. b, Compared with controls, the offspring of mice treated with poly(I:C) show a PPI deficit at a prepulse level of 85 dB. Coinjection with anti-IL-6 prevents this deficit. The PPI of offspring of mice given coinjections of poly(I:C) and anti-IFNγ or anti-IL-1β are not significantly different from control or poly(I:C) (F(4,270) = 4.195; p < 0.005). *p < 0.001 versus control; #p < 0.05 versus poly(I:C). c, d, In the open-field test, offspring of mice treated with poly(I:C) make fewer entries than controls into the center (c) and travel less total distance (d). Offspring of mice given coinjections of anti-IL-6 enter the center as often as control mice [F(3,123) = 3.703; p < 0.05; *p < 0.05 vs control; #p < 0.05 vs poly(I:C)] and move a similar total distance [F(3,123) = 6.666; p < 0.0005; *p < 0.01 vs control; #p < 0.001 vs poly(I:C)]. Offspring of mice given coinjections of poly(I:C) and anti-IFNγ are not significantly different from controls or poly(I:C). e, Tracks recorded during the open-field session demonstrate increased thigmotaxis in offspring of poly(I:C)-treated mice compared with offspring of mice given coinjections of an IL-6 antibody. f, In the social interaction test, control mice show a strong preference for the social chamber [defined as (percentage of time in social chamber) − (percentage of time in opposite chamber)], whereas the offspring of poly(I:C)-treated mice show no such preference. Again, the deficit is corrected by maternal administration of IL-6 antibody (F(3,50) = 4.244; p < 0.01). *p < 0.05 versus control; #p < 0.05 versus poly(I:C).
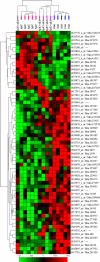
Figure 3.
Unsupervised clustering of microarray data show anti-IL-6 treatment rescues transcriptome changes in MIA offspring. Sixty-one genes show significant (p < 0.01) expression differences between the adult offspring of poly(I:C)-treated and saline-injected mice. Two-dimensional, unsupervised clustering of these genes (_x_-axis genes, _y_-axis samples) reveals control (Sal; blue bars), poly(I:C) (pIC; red bars), and poly(I:C) plus anti-IL-6 (pIC+aIL6; purple bars) animals cluster according to treatment, with only one pIC+aIL6 outlier. Significantly, the pIC+aIL6 animals cluster with saline-injected controls, rather than with pIC offspring. Each column represents expression values from a single animal. Genes are annotated by Affymetrix probe set/Unigene identifiers. Color intensity represents the magnitude of the gene expression change compared with the overall average intensity (green, decreased; red, increased; black, unchanged).
Similar articles
- Impact of maternal immune activation and sex on placental and fetal brain cytokine and gene expression profiles in a preclinical model of neurodevelopmental disorders.
Osman HC, Moreno R, Rose D, Rowland ME, Ciernia AV, Ashwood P. Osman HC, et al. J Neuroinflammation. 2024 May 7;21(1):118. doi: 10.1186/s12974-024-03106-7. J Neuroinflammation. 2024. PMID: 38715090 Free PMC article. - Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism.
Parker-Athill E, Luo D, Bailey A, Giunta B, Tian J, Shytle RD, Murphy T, Legradi G, Tan J. Parker-Athill E, et al. J Neuroimmunol. 2009 Dec 10;217(1-2):20-7. doi: 10.1016/j.jneuroim.2009.08.012. Epub 2009 Sep 18. J Neuroimmunol. 2009. PMID: 19766327 Free PMC article. - Intrauterine position effects in a mouse model of maternal immune activation.
Schaer R, Mueller FS, Notter T, Weber-Stadlbauer U, Meyer U. Schaer R, et al. Brain Behav Immun. 2024 Aug;120:391-402. doi: 10.1016/j.bbi.2024.06.015. Epub 2024 Jun 17. Brain Behav Immun. 2024. PMID: 38897330 - Maternal IL-17A in autism.
Wong H, Hoeffer C. Wong H, et al. Exp Neurol. 2018 Jan;299(Pt A):228-240. doi: 10.1016/j.expneurol.2017.04.010. Epub 2017 Apr 25. Exp Neurol. 2018. PMID: 28455196 Free PMC article. Review. - Virus-Induced Maternal Immune Activation as an Environmental Factor in the Etiology of Autism and Schizophrenia.
Massrali A, Adhya D, Srivastava DP, Baron-Cohen S, Kotter MR. Massrali A, et al. Front Neurosci. 2022 Apr 12;16:834058. doi: 10.3389/fnins.2022.834058. eCollection 2022. Front Neurosci. 2022. PMID: 35495047 Free PMC article. Review.
Cited by
- The Microbiota, Immunoregulation, and Mental Health: Implications for Public Health.
Lowry CA, Smith DG, Siebler PH, Schmidt D, Stamper CE, Hassell JE Jr, Yamashita PS, Fox JH, Reber SO, Brenner LA, Hoisington AJ, Postolache TT, Kinney KA, Marciani D, Hernandez M, Hemmings SM, Malan-Muller S, Wright KP, Knight R, Raison CL, Rook GA. Lowry CA, et al. Curr Environ Health Rep. 2016 Sep;3(3):270-86. doi: 10.1007/s40572-016-0100-5. Curr Environ Health Rep. 2016. PMID: 27436048 Free PMC article. Review. - Regulation of microglial physiology by the microbiota.
Cook J, Prinz M. Cook J, et al. Gut Microbes. 2022 Jan-Dec;14(1):2125739. doi: 10.1080/19490976.2022.2125739. Gut Microbes. 2022. PMID: 36151874 Free PMC article. Review. - Allergic fetal priming leads to developmental, behavioral and neurobiological changes in mice.
Schwartzer JJ, Careaga M, Chang C, Onore CE, Ashwood P. Schwartzer JJ, et al. Transl Psychiatry. 2015 Apr 7;5(4):e543. doi: 10.1038/tp.2015.40. Transl Psychiatry. 2015. PMID: 25849982 Free PMC article. - Exposure to prenatal infection and risk of schizophrenia.
Brown AS. Brown AS. Front Psychiatry. 2011 Nov 23;2:63. doi: 10.3389/fpsyt.2011.00063. eCollection 2011. Front Psychiatry. 2011. PMID: 22131978 Free PMC article. - _Mycobacterium tuberculosis_-Induced Maternal Immune Activation Promotes Autism-Like Phenotype in Infected Mice Offspring.
Manjeese W, Mvubu NE, Steyn AJC, Mpofana T. Manjeese W, et al. Int J Environ Res Public Health. 2021 Apr 23;18(9):4513. doi: 10.3390/ijerph18094513. Int J Environ Res Public Health. 2021. PMID: 33922864 Free PMC article.
References
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. - PubMed
- Arad M, Atzil S, Shakhar K, Adoni A, Ben-Eliyahu S. Poly I-C induces early embryo loss in f344 rats: a potential role for NK cells. Am J Reprod Immunol. 2005;54:49–53. - PubMed
- Armario A, Hernandez J, Bluethmann H, Hidalgo J. IL-6 deficiency leads to increased emotionality in mice: evidence in transgenic mice carrying a null mutation for IL-6. J Neuroimmunol. 1998;92:160–169. - PubMed
- Aronsson F, Lannebo C, Paucar M, Brask J, Kristensson K, Karlsson H. Persistence of viral RNA in the brain of offspring to mice infected with influenza A/WSN/33 virus during pregnancy. J Neurovirol. 2002;8:353–357. - PubMed
- Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical