Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment - PubMed (original) (raw)
Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment
Karen F S Bell et al. J Neurosci. 2007.
Abstract
Synaptic integrity is now recognized as a central component of Alzheimer's disease. Surprisingly, however, the structural status of glutamatergic synapses in Alzheimer's disease is unclear, despite the fact that glutamate is the major excitatory transmitter of the CNS and has key roles in excitotoxicity and long-term potentiation. The identification of specific markers of glutamatergic neurons now allows an assessment of the structural involvement of the glutamatergic system across progressive stages of the Alzheimer's pathology, an opportunity not afforded by previously used neurochemical approaches. Glutamatergic presynaptic bouton density and dystrophic neurite abundance were quantified in midfrontal gyrus brain tissue from subjects with no cognitive impairment, mild cognitive impairment, or mild- or severe-stage Alzheimer's disease. Our study demonstrates a striking pathology-dependent pattern of glutamatergic synaptic remodeling with disease progression. Subjects with mild cognitive impairment display a paradoxical elevation in glutamatergic presynaptic bouton density, a situation akin to that observed in the cholinergic system, which then depletes and drops with disease progression. This pattern of synaptic remodeling mirrors our previous findings in transgenic animal models and is of major relevance to current transmitter-based therapeutics.
Figures
Figure 1.
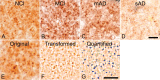
Immunohistochemical staining of the glutamatergic presynaptic bouton sites in human midfrontal gyrus tissue using antibodies directed against the glutamatergic presynaptic bouton site-specific marker vesicular glutamate transporter 1. A–D, Stainings are from subjects with no cognitive impairment (A), mild cognitive impairment (B), mild AD (C), or severe AD (D). Note the elevation in terminal number in B, taken from a subject with mild cognitive impairment, and the decreased presynaptic bouton-immunoreactivities in C and D, taken from subjects with mild and severe AD, respectively. E–G, The quantification protocol used to determine glutamatergic presynaptic bouton density in the midfrontal gyrus. Original digital images as shown in E were transformed into a file type that increases the computer's accuracy of detection (F). This transformed file format is then quantified by the computer using precise inclusion and exclusion criteria (as described in Materials and Methods) to accurately detect the elements of interest. G, The quantified image, in which dark blue coloration indicates those elements that were quantified, and light blue coloration indicates elements that failed to meet the required criteria and were hence omitted. Quantified data are then tallied and yielded in number format. Scale bars: D (for A–D), G (for E–G), 10 μm.
Figure 2.
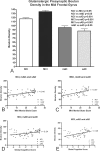
Graphic depiction of the relationship between glutamatergic presynaptic bouton density (number of presynaptic boutons per 1000 μm2 area) and cognitive ability. A, Mean midfrontal gyrus glutamatergic presynaptic bouton densities in subjects grouped by cognitive ability (NCI, MCI, mAD, and sAD). Means were compared by ANOVA followed by post hoc Tukey's test. B–E, Positive associations between increased glutamatergic presynaptic bouton density and cognitive performance for both the Mini Mental State Examination (B, C) and Global Cognitive Test score (D, E). Data were grouped by cognitive ability as NCI, mAD, and sAD (B, D) or MCI, mAD, and sAD (C, E). Note that cognitive performance declines with decreasing glutamatergic presynaptic bouton density across all groupings and test types.
Figure 3.
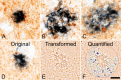
Double-immunohistochemical staining of glutamatergic dystrophic neurites (brown) and amyloid plaques (blue) in the midfrontal gyrus brain region. Note the predominant localization of dystrophic neurites to the inner areas of amyloid plaques, as opposed to the plaque periphery. Note also the increase in abundance of glutamatergic dystrophic neurites with increasing plaque size (A–C). D–F show the manner in which the relative abundance (total area of occupation) of glutamatergic dystrophic neurites was determined. E, The transformed file, which, as previously described, increases the computer's accuracy of detection. F, The encircled quantified area, in which dark blue coloration shows elements that met the required inclusion and exclusion criteria (size, color, intensity, saturation, and roundness) and were therefore included in the quantification. The light blue coloration shows elements that failed to match the necessary criteria and that were therefore excluded. Note the close level of overlap between computer detection and dystrophic neurite abundance, as well as the omission of smaller terminals or plaque fragments. Scale bar, 10 μm.
Figure 4.
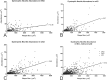
Graphic depiction of the association between glutamatergic dystrophic neurite abundance and amyloid plaque size in the midfrontal gyrus of subjects with MCI (A), mAD (B), sAD (C), or all three subject groups combined (MCI, mAD, and sAD; D). Note the positive association between increasing plaque size and increasing glutamatergic dystrophic neurite abundance in all three groups. Note also that amyloid plaque size appears to increase with disease progression, as does the strength of the Pearson's correlation, thus suggesting that increasing levels of fibrillar Aβ correlate with increased glutamatergic terminal toxicity.
Similar articles
- The impact of Abeta-plaques on cortical cholinergic and non-cholinergic presynaptic boutons in alzheimer's disease-like transgenic mice.
Hu L, Wong TP, Côté SL, Bell KF, Cuello AC. Hu L, et al. Neuroscience. 2003;121(2):421-32. doi: 10.1016/s0306-4522(03)00394-4. Neuroscience. 2003. PMID: 14522000 - Structural involvement of the glutamatergic presynaptic boutons in a transgenic mouse model expressing early onset amyloid pathology.
Bell KF, de Kort GJ, Steggerda S, Shigemoto R, Ribeiro-da-Silva A, Cuello AC. Bell KF, et al. Neurosci Lett. 2003 Dec 19;353(2):143-7. doi: 10.1016/j.neulet.2003.09.027. Neurosci Lett. 2003. PMID: 14664921 - Presynaptic proteins complexin-I and complexin-II differentially influence cognitive function in early and late stages of Alzheimer's disease.
Ramos-Miguel A, Sawada K, Jones AA, Thornton AE, Barr AM, Leurgans SE, Schneider JA, Bennett DA, Honer WG. Ramos-Miguel A, et al. Acta Neuropathol. 2017 Mar;133(3):395-407. doi: 10.1007/s00401-016-1647-9. Epub 2016 Nov 19. Acta Neuropathol. 2017. PMID: 27866231 Free PMC article. - Amyloid Plaques of Alzheimer's Disease as Hotspots of Glutamatergic Activity.
Ovsepian SV, O'Leary VB, Zaborszky L, Ntziachristos V, Dolly JO. Ovsepian SV, et al. Neuroscientist. 2019 Aug;25(4):288-297. doi: 10.1177/1073858418791128. Epub 2018 Jul 27. Neuroscientist. 2019. PMID: 30051750 Free PMC article. Review. - Pathology of presynaptic proteins in Alzheimer's disease: more than simple loss of terminals.
Honer WG. Honer WG. Neurobiol Aging. 2003 Dec;24(8):1047-62. doi: 10.1016/j.neurobiolaging.2003.04.005. Neurobiol Aging. 2003. PMID: 14643376 Review.
Cited by
- Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease.
Takahashi K, Foster JB, Lin CL. Takahashi K, et al. Cell Mol Life Sci. 2015 Sep;72(18):3489-506. doi: 10.1007/s00018-015-1937-8. Epub 2015 Jun 2. Cell Mol Life Sci. 2015. PMID: 26033496 Free PMC article. Review. - Transgenic autoinhibition of p21-activated kinase exacerbates synaptic impairments and fronto-dependent behavioral deficits in an animal model of Alzheimer's disease.
Bories C, Arsenault D, Lemire M, Tremblay C, De Koninck Y, Calon F. Bories C, et al. Aging (Albany NY). 2017 May 16;9(5):1386-1403. doi: 10.18632/aging.101239. Aging (Albany NY). 2017. PMID: 28522792 Free PMC article. - Tau-mediated synaptic damage in Alzheimer's disease.
Jadhav S, Cubinkova V, Zimova I, Brezovakova V, Madari A, Cigankova V, Zilka N. Jadhav S, et al. Transl Neurosci. 2015 Oct 23;6(1):214-226. doi: 10.1515/tnsci-2015-0023. eCollection 2015. Transl Neurosci. 2015. PMID: 28123806 Free PMC article. Review. - Hippocampal alterations in glutamatergic signaling during amyloid progression in AβPP/PS1 mice.
Hascup KN, Findley CA, Sime LN, Hascup ER. Hascup KN, et al. Sci Rep. 2020 Sep 2;10(1):14503. doi: 10.1038/s41598-020-71587-6. Sci Rep. 2020. PMID: 32879385 Free PMC article. - Restored glial glutamate transporter EAAT2 function as a potential therapeutic approach for Alzheimer's disease.
Takahashi K, Kong Q, Lin Y, Stouffer N, Schulte DA, Lai L, Liu Q, Chang LC, Dominguez S, Xing X, Cuny GD, Hodgetts KJ, Glicksman MA, Lin CL. Takahashi K, et al. J Exp Med. 2015 Mar 9;212(3):319-32. doi: 10.1084/jem.20140413. Epub 2015 Feb 23. J Exp Med. 2015. PMID: 25711212 Free PMC article.
References
- Barnes LL, Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Gender, cognitive decline, and risk of AD in older persons. Neurology. 2003;60:1777–1781. - PubMed
- Bell KF, de Kort GJ, Steggerda S, Shigemoto R, Ribeiro-da-Silva A, Cuello AC. Structural involvement of the glutamatergic presynaptic boutons in a transgenic mouse model expressing early onset amyloid pathology. Neurosci Lett. 2003;353:143–147. - PubMed
- Bell KF, Ducatenzeiler A, Ribeiro-da-Silva A, Duff K, Bennett DA, Cuello AC. The amyloid pathology progresses in a neurotransmitter-specific manner. Neurobiol Aging. 2006;27:1644–1657. - PubMed
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. - PubMed
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Berry-Kravis E, Bienias JL. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology. 2003;60:246–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources