Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome - PubMed (original) (raw)
Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome
Michael Ogier et al. J Neurosci. 2007.
Abstract
Rett syndrome (RTT) is caused by loss-of-function mutations in the gene encoding methyl-CpG-binding protein 2 (MeCP2). Although MeCP2 is thought to act as a transcriptional repressor of brain-derived neurotrophic factor (BDNF), Mecp2 null mice, which develop an RTT-like phenotype, exhibit progressive deficits in BDNF expression. These deficits are particularly significant in the brainstem and nodose cranial sensory ganglia (NGs), structures critical for cardiorespiratory homeostasis, and may be linked to the severe respiratory abnormalities characteristic of RTT. Therefore, the present study used Mecp2 null mice to further define the role of MeCP2 in regulation of BDNF expression and neural function, focusing on NG neurons and respiratory control. We find that mutant neurons express significantly lower levels of BDNF than wild-type cells in vitro, as in vivo, under both depolarizing and nondepolarizing conditions. However, BDNF levels in mutant NG cells can be increased by chronic depolarization in vitro or by treatment of Mecp2 null mice with CX546, an ampakine drug that facilitates activation of glutamatergic AMPA receptors. Ampakine-treated Mecp2 null mice also exhibit marked functional improvement, characterized by restoration of normal breathing frequency and minute volume. These data demonstrate that BDNF expression remains plastic in Mecp2 null mice and raise the possibility that ampakine compounds could be of therapeutic value in the treatment of RTT.
Figures
Figure 1.
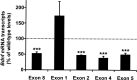
Mecp2 null mutation is associated with decreased expression of specific Bdnf transcripts in nodose neurons. Bdnf transcript levels in intact NG from wild-type and Mecp2 null mice were determined using qRT-PCR. The Bdnf gene has a complex structure in which multiple promoters drive the expression of different mRNA isoforms containing alternative noncoding 5′ exons spliced to a common downstream coding exon [exon 8; nomenclature of Liu et al. (2006)]. Total Bdnf mRNA levels (Exon 8), as well as transcripts containing exons 2, 4, and 5 were markedly decreased in mutant NG compared with wild type, whereas transcripts containing exon 1 were expressed at levels that were not significantly different from wild type. Results are the mean ± SEM (n = 4). ***p < 0.001, ANOVA I with post hoc Tukey's test.
Figure 2.
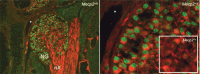
MeCP2 protein is expressed in nodose neurons. Left, Double immunostaining for MeCP2 (green) and β-tubulin III (red) in the newborn wild-type (Mecp2 +/y) mouse NG. nX, Vagal nerve. Right, Higher magnification of the same section shown on the left, illustrating the concentration of MeCP2-immunoreactive protein in heterochromatin foci. The inset shows that the MeCP2 antibody used in these studies does not produce any specific staining in the NG from a Mecp2 null mouse (Mecp2 −/y). The asterisk represents an anatomical landmark shared by both panels.
Figure 3.
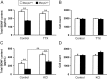
BDNF levels are depressed in P35 Mecp2 −/y NG neurons under resting and depdarizing conditions. A, C, Summary data showing that BDNF content is decreased by 40–50% in NG cultures from Mecp2 null mutants, regardless of the activity state of the cells [i.e., electrically silent (A; treated with TTX) or chronic depolarization (C, treated with KCl)]. Results show that KCl treatment can increase the BDNF level in mutant cells as in wild-type controls. B, D, Neuron survival was unaffected by either TTX (B) or KCl (D). Results are the mean ± SEM (n = 6). **p < 0.01, ANOVA I with post hoc Tukey's test.
Figure 4.
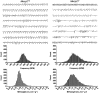
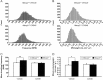
Mecp2 null mice exhibit a Rett-like respiratory phenotype at 5 weeks of age (P35). Representative plethysmographic recordings from wild-type (Mecp2 +/y) and Mecp2 null (Mecp2 −/y) mice are shown. Each trace is 10 s quiet breathing in room air. The bottom graphs are frequency histograms from control (compilation of 9776 breath cycles) and mutant (compilation of 6065 breath cycles) mice showing the higher incidence of fast breaths in mutant mice compared with controls, along with a shift to higher values of minute volume/weight. BPM, Breaths per minute; MV, minute volume.
Figure 5.
Chronic treatment with CX546 restores normal breathing frequency and minute volume/weight in P35 Mecp2 null mice. A, B, Representative histograms of breathing frequency (A) and minute volume/weight (B) from two mutant mice, one treated with vehicle (9227 breath cycles) and one treated with CX546 (8393 breath cycles), showing that drug treatment (40 mg/kg, b.i.d for 3 d) decreases episodes of high breathing frequency and minute volume/weight. C, D, Summary data for breathing frequency (C) and minute volume/weight (D) for all animals. Ampakine treatment completely restores wild-type frequency and minute volume/weight in mutant animals and has no effect in wild types. Results are the mean ± SEM (n = 8 for vehicle-treated wild types; n = 7 for CX546-treated wild types; n = 8 for vehicle-treated mutants; n = 9 for CX546-treated mutants). *p < 0.05; **p < 0.01, ANOVA I with post hoc Tukey's test. BPM, Breaths per minute.
Similar articles
- The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression.
Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. Chang Q, et al. Neuron. 2006 Feb 2;49(3):341-8. doi: 10.1016/j.neuron.2005.12.027. Neuron. 2006. PMID: 16446138 - A BDNF loop-domain mimetic acutely reverses spontaneous apneas and respiratory abnormalities during behavioral arousal in a mouse model of Rett syndrome.
Kron M, Lang M, Adams IT, Sceniak M, Longo F, Katz DM. Kron M, et al. Dis Model Mech. 2014 Sep;7(9):1047-55. doi: 10.1242/dmm.016030. Dis Model Mech. 2014. PMID: 25147297 Free PMC article. - Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice.
Kline DD, Ogier M, Kunze DL, Katz DM. Kline DD, et al. J Neurosci. 2010 Apr 14;30(15):5303-10. doi: 10.1523/JNEUROSCI.5503-09.2010. J Neurosci. 2010. PMID: 20392952 Free PMC article. - Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome.
Filosa S, Pecorelli A, D'Esposito M, Valacchi G, Hajek J. Filosa S, et al. Free Radic Biol Med. 2015 Nov;88(Pt A):81-90. doi: 10.1016/j.freeradbiomed.2015.04.019. Epub 2015 May 8. Free Radic Biol Med. 2015. PMID: 25960047 Review. - Brain-derived neurotrophic factor and Rett syndrome.
Katz DM. Katz DM. Handb Exp Pharmacol. 2014;220:481-95. doi: 10.1007/978-3-642-45106-5_18. Handb Exp Pharmacol. 2014. PMID: 24668484 Review.
Cited by
- Progress toward treatments for synaptic defects in autism.
Delorme R, Ey E, Toro R, Leboyer M, Gillberg C, Bourgeron T. Delorme R, et al. Nat Med. 2013 Jun;19(6):685-94. doi: 10.1038/nm.3193. Epub 2013 Jun 6. Nat Med. 2013. PMID: 23744158 Review. - Pattern sensitivity of ampakine-hypoxia interactions for evoking phrenic motor facilitation in anesthetized rat.
Thakre PP, Fuller DD. Thakre PP, et al. J Neurophysiol. 2024 Feb 1;131(2):216-224. doi: 10.1152/jn.00315.2023. Epub 2023 Dec 20. J Neurophysiol. 2024. PMID: 38116608 - Autism spectrum disorder causes, mechanisms, and treatments: focus on neuronal synapses.
Won H, Mah W, Kim E. Won H, et al. Front Mol Neurosci. 2013 Aug 5;6:19. doi: 10.3389/fnmol.2013.00019. eCollection 2013. Front Mol Neurosci. 2013. PMID: 23935565 Free PMC article. - Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders.
Scattoni ML, Crawley J, Ricceri L. Scattoni ML, et al. Neurosci Biobehav Rev. 2009 Apr;33(4):508-15. doi: 10.1016/j.neubiorev.2008.08.003. Epub 2008 Aug 13. Neurosci Biobehav Rev. 2009. PMID: 18771687 Free PMC article. Review. - The common BDNF polymorphism may be a modifier of disease severity in Rett syndrome.
Zeev BB, Bebbington A, Ho G, Leonard H, de Klerk N, Gak E, Vecsler M, Christodoulou J. Zeev BB, et al. Neurology. 2009 Apr 7;72(14):1242-7. doi: 10.1212/01.wnl.0000345664.72220.6a. Neurology. 2009. PMID: 19349604 Free PMC article.
References
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
- Armstrong DD. Neuropathology of Rett syndrome. Ment Retard Dev Disabil Res Rev. 2002;8:72–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases