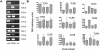TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells - PubMed (original) (raw)
TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells
Tom A Barr et al. Eur J Immunol. 2007 Nov.
Abstract
In addition to their role in humoral immunity, B lymphocytes are important antigen-presenting cells (APC). In the same way as other APC, B cells make cytokines upon activation and have the potential to modulate T cell responses. In this study, we investigated which mouse B cell subsets are the most potent cytokine producers, and examined the role of Toll-like receptors (TLR) in the control of secretion of IL-6, IL-10, IL-12 and IFN-gamma by B cells. Production of some cytokines was restricted to particular subsets. Marginal zone and B1 cells were the predominant source of B cell IL-10 in the spleen. Conversely, follicular B cells were found to express IFN-gamma mRNA directly ex vivo. The nature of the activating stimulus dramatically influenced the cytokine made by B cells. Thus, in response to combined TLR stimulation, or via phorbol esters, IFN-gamma was secreted. IL-10 was elicited by T-dependent activation or stimulation through TLR2, 4 or 9. This pattern of cytokine expression contrasts with that elicited from dendritic cells. QRT-PCR array data indicate that this may be due to differential expression of TLR signalling molecules, effectors and adaptors. Our data highlight the potentially unique nature of immune modulation when B cells act as APC.
Figures
Figure 1
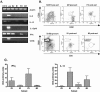
Cytokine mRNA expression by B cells. B cells were purified by CD19+ve MACS, then FACS sorted according to expression of cell surface markers. (A) RT-PCR analysis of mRNA extracted from cell sorts; +ve is the positive control (unsorted splenocytes). Semi-quantitative RT-PCR for β-actin, IL-6, IL-10, IL-12p40 and CD3ξ are shown. (B) Flow cytometry-based sorting of B cell subsets on CD19+ve pre-sorted splenocytes. Upper panels show MZ/FO sorting based on expression of CD21 and CD23. Lower panels show B1/B2 cell sorting based on expression of CD5 and B220. (C) QRT-PCR analysis of mRNA from MZ/FO and B1/B2 cell sorts. cDNA concentrations, relative to the β-actin housekeeping control gene, are indicated for IFN-γ and IL-10. Error bars indicate SEM on PCR performed on two separate RNA samples, each extracted from pooled cells from five mice.
Figure 2
B cell expression of TLR mRNA. (A) RT-PCR performed on mRNA extracted from highly purified CD19+ B unsorted splenocytes (+ve) and negative control (H2O). (B) Differential expression of TLR mRNA by MZ, FO, B1 and B2 B cell subsets. All values are given as DNA concentrations relative to the expression levels of the β-actin housekeeping gene. Results shown are based on the mean values calculated from two separate reactions performed on pooled cells from five mice per group.
Figure 3
Activation of B cells through TLR stimulation. B cells were cultured overnight with a range of TLR agonists as indicated at the top of the graph. Cells were then stained with mAb against a range of B cell activation markers, as indicated on the left. For each plot, the mean fluorescent intensity is plotted against % of maximum, with the filled grey histogram representing staining for unactivated B cells (incubated overnight in the absence of any TLR stimulus) and the open histogram representing staining of cells activated in the presence of the corresponding TLR agonist. (A) Splenic CD19+ve B cells. (B) MZ B cells (CD19+ve, B220+ve, CD21high, CD23int/low). (C) FO B cells (CD19+ve, B220+ve, CD21+ve, CD23high). Representative data from three separate experiments are shown.
Figure 4
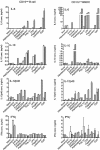
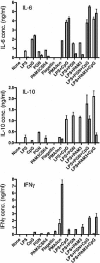
Cytokine production by DC and B cells stimulated with TLR agonists. Highly purified B cells and DC were cultured at 2 × 106 cells/mL in the presence of a range of stimuli, either alone (white bars), or in the presence of anti-CD40 antibody (grey bars). After 5 days, culture supernatants were harvested and assayed by ELISA to determine the concentrations of IL-6, IL-10, IL-12p40 and IFN-γ. Data shown are based upon triplicate cultures of pooled cells sorted from four mice and are representative of four separate experiments. Error bars represent SEM. The limits of detection for each ELISA were as follows: IL-6 = 0.3 ng/mL; IL-10 = 0.1 ng/mL; IL-12p40 = 0.1 ng/mL; and IFN-γ = 0.8 ng/mL.
Figure 5
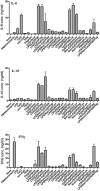
Combined TLR stimulation of B cells. CD19 MACS-purified B cells were cultured with defined combinations of TLR agonists as indicated on the x axis at 2 × 106 cells/mL. After 5 days of in vitro stimulation, supernatants were assayed for levels of IL-6, IL-10 and IFN-γ. Data shown are based on triplicate cultures of pooled cells from groups of four mice and are representative of four separate experiments. Error bars indicate SEM. Limits of detection were as follows: IL-6 = 0.3 ng/mL; IL-10 = 0.1 ng/mL; and IFN-γ = 0.8 ng/mL.
Figure 6
TLR-mediated cytokine production by purified B cell subsets. B cells isolated by a combination of CD19 MACS selection and FACS were cultured at 1 × 106 cells/mL with stimuli known to induce cytokine secretion by whole CD19+ve B cells, as indicated on the x axes. After 5 days in culture, supernatants were harvested and assayed to determine concentrations of IL-6, IL-10 and IFN-γ by ELISA. For each graph, MZ B cells are represented by white bars and FO B cells by grey bars. Data presented are based on duplicate cultures of cells pooled from four mice and representative of four separate experiments. Error bars represent SEM. Limits of detection were as follows: IL-6 = 0.3 ng/mL; IL-10 = 0.1 ng/mL; and IFN-γ = 0.8 ng/mL.
Similar articles
- Role of marginal zone B lymphocytes in invariant NKT cell activation.
Bialecki E, Paget C, Fontaine J, Capron M, Trottein F, Faveeuw C. Bialecki E, et al. J Immunol. 2009 May 15;182(10):6105-13. doi: 10.4049/jimmunol.0802273. J Immunol. 2009. PMID: 19414762 - Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates.
Ketloy C, Engering A, Srichairatanakul U, Limsalakpetch A, Yongvanitchit K, Pichyangkul S, Ruxrungtham K. Ketloy C, et al. Vet Immunol Immunopathol. 2008 Sep 15;125(1-2):18-30. doi: 10.1016/j.vetimm.2008.05.001. Epub 2008 May 8. Vet Immunol Immunopathol. 2008. PMID: 18571243 - Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets.
Gururajan M, Jacob J, Pulendran B. Gururajan M, et al. PLoS One. 2007 Sep 12;2(9):e863. doi: 10.1371/journal.pone.0000863. PLoS One. 2007. PMID: 17848994 Free PMC article. - Does helminth activation of toll-like receptors modulate immune response in multiple sclerosis patients?
Correale J, Farez MF. Correale J, et al. Front Cell Infect Microbiol. 2012 Aug 24;2:112. doi: 10.3389/fcimb.2012.00112. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22937527 Free PMC article. Review. - Tolls for B cells.
Fillatreau S, Manz RA. Fillatreau S, et al. Eur J Immunol. 2006 Apr;36(4):798-801. doi: 10.1002/eji.200636040. Eur J Immunol. 2006. PMID: 16572389 Review.
Cited by
- Antitumor Efficacy of Radiation plus Immunotherapy Depends upon Dendritic Cell Activation of Effector CD8+ T Cells.
Dovedi SJ, Lipowska-Bhalla G, Beers SA, Cheadle EJ, Mu L, Glennie MJ, Illidge TM, Honeychurch J. Dovedi SJ, et al. Cancer Immunol Res. 2016 Jul;4(7):621-630. doi: 10.1158/2326-6066.CIR-15-0253. Epub 2016 May 30. Cancer Immunol Res. 2016. PMID: 27241845 Free PMC article. - Neddylation plays an important role in the regulation of murine and human dendritic cell function.
Mathewson N, Toubai T, Kapeles S, Sun Y, Oravecz-Wilson K, Tamaki H, Wang Y, Hou G, Sun Y, Reddy P. Mathewson N, et al. Blood. 2013 Sep 19;122(12):2062-73. doi: 10.1182/blood-2013-02-486373. Epub 2013 Jul 17. Blood. 2013. PMID: 23863900 Free PMC article. - Antigen Presenting Properties of a Myeloid Dendritic-Like Cell in Murine Spleen.
Hey YY, O'Neill HC. Hey YY, et al. PLoS One. 2016 Sep 21;11(9):e0162358. doi: 10.1371/journal.pone.0162358. eCollection 2016. PLoS One. 2016. PMID: 27654936 Free PMC article. - Unraveling effector functions of B cells during infection: the hidden world beyond antibody production.
León B, Ballesteros-Tato A, Misra RS, Wojciechowski W, Lund FE. León B, et al. Infect Disord Drug Targets. 2012 Jun;12(3):213-21. doi: 10.2174/187152612800564437. Infect Disord Drug Targets. 2012. PMID: 22394173 Free PMC article. Review. - DAMPening inflammation by modulating TLR signalling.
Piccinini AM, Midwood KS. Piccinini AM, et al. Mediators Inflamm. 2010;2010:672395. doi: 10.1155/2010/672395. Epub 2010 Jul 13. Mediators Inflamm. 2010. PMID: 20706656 Free PMC article. Review.
References
- Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54(Pt 1):1–13. - PubMed
- Medzhitov R, Janeway CA., Jr. Innate immunity: Impact on the adaptive immune response. Curr. Opin. Immunol. 1997;9:4–9. - PubMed
- Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: Endogenous activators of dendritic cells. Nat. Med. 1999;5:1249–1255. - PubMed
- Kodaira Y, Nair SK, Wrenshall LE, Gilboa E, Platt JL. Phenotypic and functional maturation of dendritic cells mediated by heparan sulfate. J. Immunol. 2000;165:1599–1604. - PubMed
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting Edge: Heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 2000;164:558–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases