The neuropathology of autism - PubMed (original) (raw)
Review
The neuropathology of autism
Manuel F Casanova. Brain Pathol. 2007 Oct.
Abstract
Autism is a brain disorder characterized by abnormalities in how a person relates and communicates to others. Both post-mortem and neuroimaging studies indicate the presence of increased brain volume and, in some cases, an altered gray/white matter ratio. Contrary to established gross findings there is no recognized microscopic pathology to autism. Early studies provided multiple leads none of which have been validated. Clinicopathological associations have been difficult to sustain when considering possible variables such as use of medications, seizures, mental retardation and agonal/pre-agonal conditions. Research findings suggest widespread cortical abnormalities, lack of a vascular component and an intact blood-brain barrier. Many of the previously mentioned findings can be explained in terms of a mini-columnopathy. The significance of future controlled studies should be judged based on their explanatory powers; that is, how well do they relate to brain growth abnormalities and/or provide useful clinicopathological correlates.
Figures
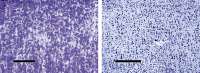
Figure 1
Radial organization of the human cerebral cortex as seen in 35‐µm sections, stained with cresyl violet. The linear appearance of the ontogenetic cell column is obscured late in gestation (left), although some vertical, striped texture is still evident. Visible minicolumnar structure re‐emerges as neuropil in the periphery of columns expands, and persists throughout life (right). Micrographs are of human temporal lobe at 32 weeks of gestation and 50 years of age, respectively. Scale bars measure 100 µm (left) and 300 µm (right), respectively.
Figure 2
The method of Schleicher et al (111) estimates the local Gray Level Index (GLI), or proportion of Nissl‐stained area. A micrograph (bottom, in false color) is segmented into two classes such that stained area is labeled 1 and unstained area (ie, background or neuropil) is labeled 0. The GLI (top) is computed by smoothing the segmented image using a kernel with oblong shape and a long axis oriented parallel to the mini‐columns. The end result is a set of summary statistics including the distance between the ridges (light blue) seen in the GLI profile, their width and their height.
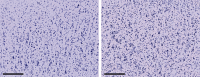
Figure 3
The Boolean germ‐grain model supposes that a spatial pattern, in this case the Nissl‐stained area in a segmented micrograph, is the union of random closed sets (grains) drawn from some distribution and positioned at random points in space (the germ process). This model is a simplification in that it does not account for clustering of the grains, eg the minicolumnar structure. Under these assumptions, the mean grain area (Ā) and perimeter (Ū), along with the intensity (λ) of the germ process, completely determine the spatial pattern, providing for a model‐based estimate of neuronal cross‐section and density. These quantities are found to be reduced and increased, respectively, in autism. Left: cortical area 9, right hemisphere, lamina III from a 25‐year‐old man without autism. Ā = 123 µm2; λ = 0.0052µm−2. Right: the same region from a 24‐year‐old autistic man. Ā = 89.1 µm2; λ = 0.0069 µm−2. Scale bars measure 200 µm.
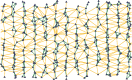
Figure 4
The Delaunay triangulation is a graph with vertices belonging to a given point set. Three points are mutually joined by edges of the graph when the circle through those points contains no other point in its interior. This figure illustrates the construction for a randomly generated set of points, clustered into vertical columns. Some edges of the triangulation have been omitted because of boundary effects. For the meaning of the coloring, see Figure 5.
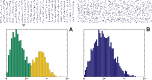
Figure 5
When a point set exhibits clustering (A), edges of the Delaunay triangulation comprise those between two points in the same cluster, and those between two points in different clusters. If very long edges between points near the boundary of the region of interest are excluded (ie, those omitted in Figure 4), the distribution of edge lengths can be modeled as a mixture of intracluster and intercluster length distributions with means m near and m far, respectively. Edges likely to join two points within the same cluster, as determined by thresholding the distribution of edge lengths, are shown in green, while those likely to be intercluster edges are shown in yellow. In contrast, the Delaunay triangulation of a completely random arrangement of points (B) has a unimodal distribution. The horizontal scale of the edge length histograms is in units of w, the horizontal distance between clustering centers in (A).
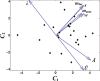
Figure 6
Pairwise differences (normal autistic) in microanatomical parameters from four cortical areas in a sample of six autistic patients and six matched comparison subjects (16). For illustration, differences in each quantity have been normalized to unit standard deviation. The first two principal components C 1 and C 2 have been plotted. Arrows indicate the relative magnitude and direction of each normalized parameter, projected onto the (C 1, C 2) plane. w: minicolumnar width; s: mean distance between neurons within a mini‐column; Ā, Ū, λ: Boolean model parameters (Figure 3); m near, m far: mean within‐cluster and between‐cluster edge lengths (Figure 5).
Similar articles
- The neurobiology of autism.
Pardo CA, Eberhart CG. Pardo CA, et al. Brain Pathol. 2007 Oct;17(4):434-47. doi: 10.1111/j.1750-3639.2007.00102.x. Brain Pathol. 2007. PMID: 17919129 Free PMC article. Review. - The autistic brain: birth through adulthood.
Courchesne E, Redcay E, Kennedy DP. Courchesne E, et al. Curr Opin Neurol. 2004 Aug;17(4):489-96. doi: 10.1097/01.wco.0000137542.14610.b4. Curr Opin Neurol. 2004. PMID: 15247547 Review. - Autism--the quest continues: reflections on the Novartis symposium on autism, June 2002.
Frith U. Frith U. Neuroreport. 2002 Oct 7;13(14):1703-5. doi: 10.1097/00001756-200210070-00002. Neuroreport. 2002. PMID: 12395107 Review. No abstract available. - The new neurobiology of autism: cortex, connectivity, and neuronal organization.
Minshew NJ, Williams DL. Minshew NJ, et al. Arch Neurol. 2007 Jul;64(7):945-50. doi: 10.1001/archneur.64.7.945. Arch Neurol. 2007. PMID: 17620483 Free PMC article. Review. - Large brains in autism: the challenge of pervasive abnormality.
Herbert MR. Herbert MR. Neuroscientist. 2005 Oct;11(5):417-40. doi: 10.1177/0091270005278866. Neuroscientist. 2005. PMID: 16151044 Review.
Cited by
- Developmental changes in the spatial organization of neurons in the neocortex of humans and common chimpanzees.
Teffer K, Buxhoeveden DP, Stimpson CD, Fobbs AJ, Schapiro SJ, Baze WB, McArthur MJ, Hopkins WD, Hof PR, Sherwood CC, Semendeferi K. Teffer K, et al. J Comp Neurol. 2013 Dec 15;521(18):4249-59. doi: 10.1002/cne.23412. J Comp Neurol. 2013. PMID: 23839595 Free PMC article. - Neuroimaging endophenotypes in autism spectrum disorder.
Mahajan R, Mostofsky SH. Mahajan R, et al. CNS Spectr. 2015 Aug;20(4):412-26. doi: 10.1017/S1092852915000371. CNS Spectr. 2015. PMID: 26234701 Free PMC article. Review. - Autism as early neurodevelopmental disorder: evidence for an sAPPα-mediated anabolic pathway.
Lahiri DK, Sokol DK, Erickson C, Ray B, Ho CY, Maloney B. Lahiri DK, et al. Front Cell Neurosci. 2013 Jun 21;7:94. doi: 10.3389/fncel.2013.00094. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23801940 Free PMC article. - Trophoblast inclusions are significantly increased in the placentas of children in families at risk for autism.
Walker CK, Anderson KW, Milano KM, Ye S, Tancredi DJ, Pessah IN, Hertz-Picciotto I, Kliman HJ. Walker CK, et al. Biol Psychiatry. 2013 Aug 1;74(3):204-11. doi: 10.1016/j.biopsych.2013.03.006. Epub 2013 Apr 25. Biol Psychiatry. 2013. PMID: 23623455 Free PMC article. - The ontogenesis of language impairment in autism: a neuropsychological perspective.
Stefanatos GA, Baron IS. Stefanatos GA, et al. Neuropsychol Rev. 2011 Sep;21(3):252-70. doi: 10.1007/s11065-011-9178-6. Epub 2011 Aug 13. Neuropsychol Rev. 2011. PMID: 21842186 Review.
References
- Satcher D (1999) U.S. Surgeon General's Report on Mental Health, ch. 3, section 6. http://www.surgeongeneral.gov/library/mentalhealth/home.html.
- APA (2000) Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. APA: Arlington, Virginia.
- Archer D, Walz W (2000) Astrocytic subtypes and the gliotic response. In: Brain Hypoxia and Ischemia. Oehmichen M (ed.), pp. 101–113. Schmidt‐Romhild: Lubeck.
- Armstrong E, Curtis M, Buxhoeveden DP, Fregoe C, Zilles K, Casanova MF, McCarthy WF (1991) Cortical gyrification in the rhesus monkey: a test of the mechanical folding hypothesis. Cereb Cortex 1:426–432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical