Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors - PubMed (original) (raw)
Review
Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors
Pál Pacher et al. Cardiovasc Drug Rev. 2007 Fall.
Abstract
Accumulating evidence suggests that the reactive oxygen and nitrogen species are generated in cardiomyocytes and endothelial cells during myocardial ischemia/reperfusion injury, various forms of heart failure or cardiomyopathies, circulatory shock, cardiovascular aging, diabetic complications, myocardial hypertrophy, atherosclerosis, and vascular remodeling following injury. These reactive species induce oxidative DNA damage and consequent activation of the nuclear enzyme poly(ADP-ribose) polymerase 1 (PARP-1), the most abundant isoform of the PARP enzyme family. PARP overactivation, on the one hand, depletes its substrate, NAD+, slowing the rate of glycolysis, electron transport, and ATP formation, eventually leading to the functional impairment or death of the endothelial cells and cardiomyocytes. On the other hand, PARP activation modulates important inflammatory pathways, and PARP-1 activity can also be modulated by several endogenous factors such as various kinases, purines, vitamin D, thyroid hormones, polyamines, and estrogens, just to mention a few. Recent studies have demonstrated that pharmacological inhibition of PARP provides significant benefits in animal models of cardiovascular disorders, and novel PARP inhibitors have entered clinical development for various cardiovascular indications. Because PARP inhibitors can enhance the effect of anticancer drugs and decrease angiogenesis, their therapeutic potential is also being explored for cancer treatment. This review discusses the therapeutic effects of PARP inhibitors in myocardial ischemia/reperfusion injury, various forms of heart failure, cardiomyopathies, circulatory shock, cardiovascular aging, diabetic cardiovascular complications, myocardial hypertrophy, atherosclerosis, vascular remodeling following injury, angiogenesis, and also summarizes our knowledge obtained from the use of PARP-1 knockout mice in the various preclinical models of cardiovascular diseases.
Conflict of interest statement
Conflict of interest: CS is one of the founders and has been from 1998–2005 Chief Scientific Officer of Inotek, Inc., the developer of PARP inhibitors; PP owns stock in the company. The trust, established for the members of CS family, owns stock in Inotek, Inc. [Correction added after online publication Oct 3, 2007: PP replaced by CS]
Figures
FIG. 1
Triggers, mechanisms of PARP-mediated cell death, and exogenous/endogenous regulators/modulators of PARP activity. Reactive oxygen and nitrogen species (e.g., peroxynitrite)-dependent cytotoxicity in various cardiovascular diseases is mediated by a multitude of effects including lipid peroxidation, protein nitration and oxidation, DNA oxidative damage, activation of matrix metalloproteinases (MMPs), and inactivation of a series of enzymes. Mitochondrial enzymes are particularly vulnerable to attacks by peroxynitrite, leading to reduced ATP formation and induction of mitochondrial permeability transition by opening of the permeability transition pore, which dissipates the mitochondrial membrane potential (Δ_ψ_m). These events lead to cessation of electron transport and ATP formation, mitochondrial swelling, and permeabilization of the outer mitochondrial membrane, allowing the efflux of several proapoptotic molecules, including cytochrome or C and apoptosis-inducing factor (AIF). In turn, cytochrome or C and AIF activate a series of downstream effectors, which eventually result in the fragmentation of nuclear DNA. In addition to its damaging effects on mitochondria, peroxynitrite inflicts more or less severe oxidative injury to DNA, resulting in DNA strand breakage, which in turn activates the nuclear enzyme poly(ADP-ribose) polymerase (PARP). Activated PARP consumes NAD to build up poly(ADP-ribose) polymers (PAR), which are themselves rapidly metabolized by the activity of poly(ADP-ribose) glycohydrolase (PARG). Some free PAR may exit the nucleus and travel to the mitochondria, where they amplify the mitochondrial efflux of AIF (nuclear to mitochondria crosstalk). Depending on the severity of the initial insult by peroxynitrite or other oxidants, the injured cell may either recover or die. In the latter case, the cell may be executed by apoptosis in the case of moderate PTP opening and PARP activation with preservation of cellular ATP, or by necrosis in case of widespread PTP opening and PARP overactivation, leading to massive NAD consumption and collapse of cellular ATP. Various endogenous factors can influence PARP activity either by inhibiting the binding of its substrate NAD+ to the active site of the enzyme or by forming a complex with PARP. An example for the latter may include estrogen (E) and thyroid hormones (T) and for the former nicotinamide (NA), NAD+ metabolites, caffeine metabolites, and vitamin D. PARP activity can also be modulated by various kinases by phosphorylation (e.g., MAP kinases and PKC), and PARP can modulate kinase (e.g., AKT) activity. Various exogenous factors such as caffeine and its endogenously formed metabolites, theophylline, and tetracycline antibiotics may also modulate PARP activity. Overall, PARP appears to be a subject of multiple lines of endogenous regulators, and it is conceivable that the processes regulated by PARP (e.g., DNA repair and cellular NAD homeostasis) are under a similarly dynamic control by a multitude of factors and influences.
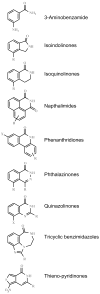
FIG. 2
Some prototypical PARP inhibitor structures.
Similar articles
- Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.
Henning RJ, Bourgeois M, Harbison RD. Henning RJ, et al. Cardiovasc Toxicol. 2018 Dec;18(6):493-506. doi: 10.1007/s12012-018-9462-2. Cardiovasc Toxicol. 2018. PMID: 29968072 Review. - Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease.
Pacher P, Szabo C. Pacher P, et al. Am J Pathol. 2008 Jul;173(1):2-13. doi: 10.2353/ajpath.2008.080019. Epub 2008 Jun 5. Am J Pathol. 2008. PMID: 18535182 Free PMC article. Review. - Pharmacological inhibition of poly(ADP-ribose) polymerase in cardiovascular disorders: future directions.
Szabó C. Szabó C. Curr Vasc Pharmacol. 2005 Jul;3(3):301-3. doi: 10.2174/1570161054368553. Curr Vasc Pharmacol. 2005. PMID: 16026326 Review. - The therapeutic potential of poly(ADP-ribose) polymerase inhibitors.
Virág L, Szabó C. Virág L, et al. Pharmacol Rev. 2002 Sep;54(3):375-429. doi: 10.1124/pr.54.3.375. Pharmacol Rev. 2002. PMID: 12223530 Review. - Poly (ADP-ribose) polymerase activation and circulatory shock.
Szabó C. Szabó C. Novartis Found Symp. 2007;280:92-103; discussion 103-7, 160-4. doi: 10.1007/0-387-36005-0_16. Novartis Found Symp. 2007. PMID: 17380790 Review.
Cited by
- Role of poly(ADP-ribosyl)ation in a 'two-hit' model of hypoxia and oxidative stress in human A549 epithelial cells in vitro.
Erdélyi K, Pacher P, Virág L, Szabó C. Erdélyi K, et al. Int J Mol Med. 2013 Aug;32(2):339-46. doi: 10.3892/ijmm.2013.1397. Epub 2013 May 29. Int J Mol Med. 2013. PMID: 23722590 Free PMC article. - Deficiency of ataxia-telangiectasia mutated kinase modulates functional and biochemical parameters of the heart in response to Western-type diet.
Wingard MC, Dalal S, Shook PL, Myers R, Connelly BA, Thewke DP, Singh M, Singh K. Wingard MC, et al. Am J Physiol Heart Circ Physiol. 2021 Jun 1;320(6):H2324-H2338. doi: 10.1152/ajpheart.00990.2020. Epub 2021 Apr 30. Am J Physiol Heart Circ Physiol. 2021. PMID: 33929897 Free PMC article. - Protective effects of PARP-1 knockout on dyslipidemia-induced autonomic and vascular dysfunction in ApoE mice: effects on eNOS and oxidative stress.
Hans CP, Feng Y, Naura AS, Zerfaoui M, Rezk BM, Xia H, Kaye AD, Matrougui K, Lazartigues E, Boulares AH. Hans CP, et al. PLoS One. 2009 Oct 13;4(10):e7430. doi: 10.1371/journal.pone.0007430. PLoS One. 2009. PMID: 19823587 Free PMC article. - Synthesis and evaluation of an AZD2461 [18F]PET probe in non-human primates reveals the PARP-1 inhibitor to be non-blood-brain barrier penetrant.
Reilly SW, Puentes LN, Schmitz A, Hsieh CJ, Weng CC, Hou C, Li S, Kuo YM, Padakanti P, Lee H, Riad AA, Makvandi M, Mach RH. Reilly SW, et al. Bioorg Chem. 2019 Mar;83:242-249. doi: 10.1016/j.bioorg.2018.10.015. Epub 2018 Oct 17. Bioorg Chem. 2019. PMID: 30390553 Free PMC article. - Attenuation of Muscle Damage, Structural Abnormalities, and Physical Activity in Respiratory and Limb Muscles following Treatment with Rucaparib in Lung Cancer Cachexia Mice.
Pérez-Peiró M, Duran X, Yélamos J, Barreiro E. Pérez-Peiró M, et al. Cancers (Basel). 2022 Jun 11;14(12):2894. doi: 10.3390/cancers14122894. Cancers (Basel). 2022. PMID: 35740560 Free PMC article.
References
- Aldinucci A, Gerlini G, Fossati S, Cipriani G, Ballerini C, Biagioli T, Pimpinelli N, Borgognoni L, Massacesi L, Moroni F, et al. A key role for poly(ADP-ribose) polymerase-1 activity during human dendritic cell maturation. J Immunol. 2007;179:305–312. - PubMed
- Azevedo LC, Pedro MA, Souza LC, de Souza HP, Janiszewski M, da Luz PL, Laurindo FR. Oxidative stress as a signaling mechanism of the vascular response to injury: The redox hypothesis of restenosis. Cardiovasc Res. 2000;47:436–445. - PubMed
- Bai P, Mabley JG, Liaudet L, Virag L, Szabo C, Pacher P. Matrix metalloproteinase activation is an early event in doxorubicin-induced cardiotoxicity. Onco Rep. 2004;11:505–508. - PubMed
- Baker CS, Dutka DP, Pagano D, Rimoldi O, Pitt M, Hall RJ, Polak JM, Bonser RS, Camici PG. Immunocytochemical evidence for inducible nitric oxide synthase and cyclooxygenase-2 expression with nitrotyrosine formation in human hibernating myocardium. Basic Res Cardiol. 2002;97:409–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous