Glial toll-like receptor signaling in central nervous system infection and autoimmunity - PubMed (original) (raw)
Review
Glial toll-like receptor signaling in central nervous system infection and autoimmunity
Pamela A Carpentier et al. Brain Behav Immun. 2008 Feb.
Abstract
Innate immunity in the CNS depends primarily on the functions of glial cells, astrocytes and microglia, which are important for the early control of pathogen replication and direct the recruitment and activation of cells of the adaptive immune system required for pathogen clearance. Efficient immune responses are required for clearance of an invading pathogen, but dysregulation of a pro-inflammatory response in the CNS could lead to the development of autoimmunity. This review summarizes the activation of toll-like receptors (TLRs) expressed on glial cells and the functional outcome of these interactions for CNS health and disease which depends on a delicate balance of the protective and toxic effects of molecules induced in the CNS following TLR ligation.
Figures
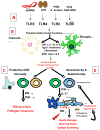
Figure 1. Schematic Representation of the Roles of TLR Signaling on Glial Cells in CNS Immunity and Disease
Glial cells, astrocytes and microglia, express a wide variety of TLRs. (A) Stimulation with TLR ligands: dsRNA (TLR3), LPS (TLR4), peptidoglycans (PGN; TLR2 with TLR1/6), and viral CpG DNA (TLR9) promotes an array of immune functions in glial cells, including, the secretion of pro-inflammatory cytokines, chemokines, type I interferons (IFN-α/β), and an increase in MHC class I and II expression. (B) Secretion of these molecules affects the adaptive immune responses in the CNS by promoting a pro-inflammatory response thereby recruiting leukocytes (macrophages, dendritic cells, and CD4+ and CD8+ T cells) to the CNS. (C) Together these functions are essential for initiating protective immunity and efficient clearance of invading pathogens from the CNS. (D) However recent evidence suggests that depending on the type of pathogen and/or route of infection, chronic activation of TLRs on glial cells may be detrimental to the host, causing neurotoxicity and oligodendrocyte or neuronal cell death. In addition to the role of TLR signaling on glial cells in protective immunity, it may also be important for the development of CNS autoimmune diseases in the absence of pathogens. The CNS inflammatory response triggered by a virus or injury leads to further CNS tissue damage. The release of sequestered myelin epitopes further propagates inflammation and self-antigen specific immune responses (Th1 and Th17 responses), thus initiating autoimmunity. TLRs on glial cells can also be activated by endogenous ligands released during tissue damage that may initiate and/or further amplify inflammation in CNS autoimmune disease.
Similar articles
- Broad expression of Toll-like receptors in the human central nervous system.
Bsibsi M, Ravid R, Gveric D, van Noort JM. Bsibsi M, et al. J Neuropathol Exp Neurol. 2002 Nov;61(11):1013-21. doi: 10.1093/jnen/61.11.1013. J Neuropathol Exp Neurol. 2002. PMID: 12430718 - Toll-like receptors in central nervous system glial inflammation and homeostasis.
Kielian T. Kielian T. J Neurosci Res. 2006 Apr;83(5):711-30. doi: 10.1002/jnr.20767. J Neurosci Res. 2006. PMID: 16541438 Free PMC article. Review. - Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases.
Li L, Acioglu C, Heary RF, Elkabes S. Li L, et al. Brain Behav Immun. 2021 Jan;91:740-755. doi: 10.1016/j.bbi.2020.10.007. Epub 2020 Oct 8. Brain Behav Immun. 2021. PMID: 33039660 Free PMC article. Review. - Satellite glial cells in human trigeminal ganglia have a broad expression of functional Toll-like receptors.
Mitterreiter JG, Ouwendijk WJD, van Velzen M, van Nierop GP, Osterhaus ADME, Verjans GMGM. Mitterreiter JG, et al. Eur J Immunol. 2017 Jul;47(7):1181-1187. doi: 10.1002/eji.201746989. Epub 2017 Jun 1. Eur J Immunol. 2017. PMID: 28508449
Cited by
- Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage.
Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Alfonso-Loeches S, et al. J Neurosci. 2010 Jun 16;30(24):8285-95. doi: 10.1523/JNEUROSCI.0976-10.2010. J Neurosci. 2010. PMID: 20554880 Free PMC article. - Cysteine Peptidase Cathepsin X as a Therapeutic Target for Simultaneous TLR3/4-mediated Microglia Activation.
Pišlar A, Nedeljković BB, Perić M, Jakoš T, Zidar N, Kos J. Pišlar A, et al. Mol Neurobiol. 2022 Apr;59(4):2258-2276. doi: 10.1007/s12035-021-02694-2. Epub 2022 Jan 23. Mol Neurobiol. 2022. PMID: 35066760 Free PMC article. - Stress- and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse.
Frank MG, Watkins LR, Maier SF. Frank MG, et al. Brain Behav Immun. 2011 Jun;25 Suppl 1(Suppl 1):S21-8. doi: 10.1016/j.bbi.2011.01.005. Epub 2011 Jan 21. Brain Behav Immun. 2011. PMID: 21256955 Free PMC article. Review. - Essential role of toll-like receptor 2 in morphine-induced microglia activation in mice.
Zhang Y, Li H, Li Y, Sun X, Zhu M, Hanley G, Lesage G, Yin D. Zhang Y, et al. Neurosci Lett. 2011 Feb 1;489(1):43-7. doi: 10.1016/j.neulet.2010.11.063. Epub 2010 Dec 2. Neurosci Lett. 2011. PMID: 21130144 Free PMC article. - Lipopolysaccharide modulates astrocytic S100B secretion: a study in cerebrospinal fluid and astrocyte cultures from rats.
Guerra MC, Tortorelli LS, Galland F, Da Ré C, Negri E, Engelke DS, Rodrigues L, Leite MC, Gonçalves CA. Guerra MC, et al. J Neuroinflammation. 2011 Oct 4;8:128. doi: 10.1186/1742-2094-8-128. J Neuroinflammation. 2011. PMID: 21970823 Free PMC article.
References
- Arimoto T, Bing G. Up-regulation of inducible nitric oxide synthase in the substantia nigra by lipopolysaccharide causes microglial activation and neurodegeneration. Neurobiol Dis. 2003;12:35–45. - PubMed
- Bailey SL, Carpentier PA, McMahon EJ, Begolka WS, Miller SD. Innate and adaptive immune responses of the central nervous system. Crit Rev Immunol. 2006;26:149–188. - PubMed
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4(+) T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. - PubMed
- Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 NS048807/NS/NINDS NIH HHS/United States
- P01 NS023349/NS/NINDS NIH HHS/United States
- P01 NS023349-210008/NS/NINDS NIH HHS/United States
- T32 AI060523/AI/NIAID NIH HHS/United States
- P01 NS023349-21/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources