Recoil and stiffening by adherent leukocytes in response to fluid shear - PubMed (original) (raw)
Recoil and stiffening by adherent leukocytes in response to fluid shear
Mark F Coughlin et al. Biophys J. 2008.
Abstract
Prolonged exposure to fluid shear stress alters leukocyte functions associated with the immune response. We examined the initial response of freshly isolated human leukocytes to fluid shear stress under high magnification. Adherent leukocytes exhibit a rapid biomechanical response to physiological levels of fluid shear stress. After passive displacement in the direction of a constant fluid shear stress, adherent leukocytes actively recoil back in the opposite direction of the fluid flow. Recoil is observed within seconds of the applied fluid shear stress. Simultaneously, fluid shear stress induces a stiffening of the cell. The immediate cell displacement in response to a step increase in fluid shear stress is greatly attenuated in subsequent steps compared to the initial fluid shear stress step. Recoil is not mediated by actin polymerization-dependent mechanisms, as cytochalasin D had no effect on this early response. However, stiffening was determined in part by an intact actin cytoskeleton. Inhibiting myosin force generation with ML-7 abolished the recoil and stiffening responses, implicating force generation by myosin as an important contributor to the early leukocyte response to fluid shear stress. This initial shear stress response may be particularly important in facilitating leukocyte attachment under sustained fluid shear stress by the flowing blood in the microcirculation.
Figures
Figure 1
Digital micrograph of a freshly isolated human leukocyte as used in the current experiments. The cell outline is indicated by a white dashed line, and the computed cell centroid is shown as a white cross. The tip of an adjacent pipette used to apply FSS is just visible to the right of the image with the direction of fluid discharge indicated by the arrow.
Figure 2
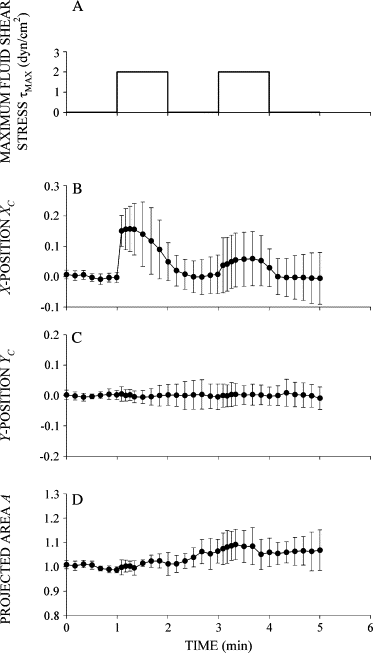
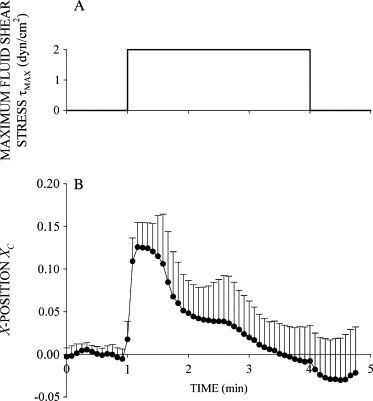
Response of human leukocytes to FSS. (A) Time course of FSS used to examine the FSS response of leukocytes. Cells were exposed to two step increases of FSS with a peak value _τ_MAX = 2 dyn/cm2 and duration of 1 min separated by 1 min. (B) Normalized _X_-position of the cell centroid _X_C shown as a function of time during exposure to FSS. The cell moved primarily in the _X_-direction, showing an immediate displacement in response to FSS followed by recoil back toward its initial position despite the presence of sustained FSS. (C) Normalized _Y_-position of the cell centroid shown as a function of time during FSS exposure. (D) Normalized projected cell area shown as a function of time during FSS exposure. Data are the mean and standard deviation of 11 observations.
Figure 3
Stress amplitude dependence of the leukocyte response to fluid shear. (A) Time course of FSS exposure. (B) Leukocyte recoil in a fluid shear field. Normalized _X_-position of the cell centroid of freshly isolated leukocytes exposed to FSS with peak values on the cell surface of _τ_MAX = 1, 2, and 4 dyn/cm2. (C) Leukocyte stiffening response. The immediate displacement in response to a step increase in FSS increased with _τ_MAX but decreased with subsequent shear stress steps. (*) indicates a statistically significant difference (p < 0.05) in immediate displacement between the first and second steps. ($) indicates a statistically significant difference (p < 0.05) in immediate displacement compared to _τ_MAX = 1 dyn/cm2. The number of observations, n, is given in the legend.
Figure 4
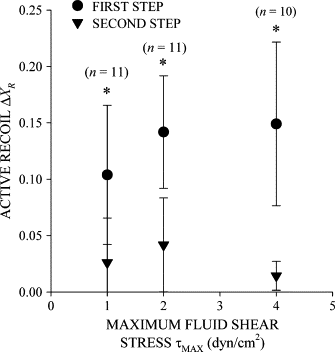
The extent of active recoil in sustained fluid shear. The active recoil Δ_X_C in the _X_-position of the cell centroid increased with the maximum FSS on the cell surface in the first shear step. The magnitude of active recoil decreased in subsequent fluid shear steps. (*) indicates statistical significance (p < 0.05) in active recoil between the first and second steps. The number of observations, n, at each maximum shear stress is given above the symbol.
Figure 5
(A) Time course of FSS application for a single step increase in fluid discharge from the pipette of 3 min duration. (B) Normalized _X_-position of the cell centroid _X_C shown as a function of time during 3 min exposure to FSS. With longer FSS application, cells eventually recoil back to their initial position and even move toward the pipette. Data are the mean and standard deviation of 10 observations.
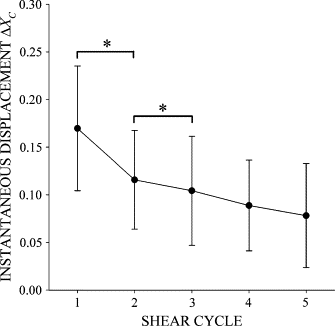
Figure 6
The instantaneous displacement of the cell centroid, Δ_X_C, in response to a step increase in FSS with a peak value of _τ_MAX = 2 dyn/cm2. (*) indicates statistical significance (p < 0.05). Data are mean and standard deviation of 17 observations.
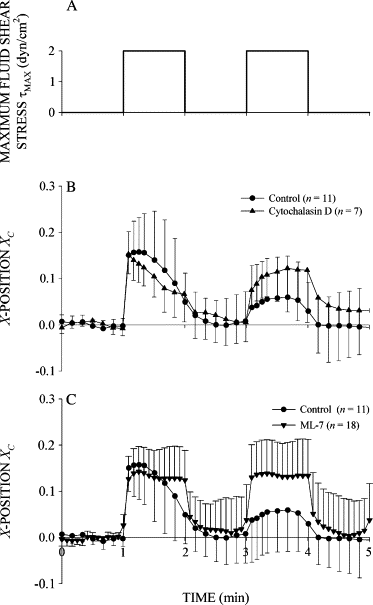
Figure 7
Cytoskeletal protein involvement in the active recoil and stiffening responses. (A) Time course of shear stress. (B) Normalized _X_-position of control cells and cells pretreated with the actin filament disrupting drug cytochalasin D at 1 mM. (C) Normalized _X_-position of control cells and cells pretreated with the myosin light chain kinase inhibitor ML-7 at 50 _μ_M. The number of observations, n, is given in the legend.
Similar articles
- L-selectin shear thresholding modulates leukocyte secondary capture.
Paschall CD, Lawrence MB. Paschall CD, et al. Ann Biomed Eng. 2008 Apr;36(4):622-31. doi: 10.1007/s10439-008-9468-1. Epub 2008 Feb 26. Ann Biomed Eng. 2008. PMID: 18299990 Free PMC article. - The fluid shear stress distribution on the membrane of leukocytes in the microcirculation.
Sugihara-Seki M, Schmid-Schönbein GW. Sugihara-Seki M, et al. J Biomech Eng. 2003 Oct;125(5):628-38. doi: 10.1115/1.1611515. J Biomech Eng. 2003. PMID: 14618922 - Mechanisms for flow-enhanced cell adhesion.
Zhu C, Yago T, Lou J, Zarnitsyna VI, McEver RP. Zhu C, et al. Ann Biomed Eng. 2008 Apr;36(4):604-21. doi: 10.1007/s10439-008-9464-5. Epub 2008 Feb 26. Ann Biomed Eng. 2008. PMID: 18299992 Free PMC article. Review. - Leukocyte fluid shear response in the presence of glucocorticoid.
Fukuda S, Mitsuoka H, Schmid-Schönbein GW. Fukuda S, et al. J Leukoc Biol. 2004 Apr;75(4):664-70. doi: 10.1189/jlb.1003464. Epub 2004 Jan 14. J Leukoc Biol. 2004. PMID: 14726499 - Mechanotransduction in leukocyte activation: a review.
Makino A, Shin HY, Komai Y, Fukuda S, Coughlin M, Sugihara-Seki M, Schmid-Schönbein GW. Makino A, et al. Biorheology. 2007;44(4):221-49. Biorheology. 2007. PMID: 18094448 Review.
Cited by
- Effects of Mechanical Stress on Endothelial Cells In Situ and In Vitro.
Katoh K. Katoh K. Int J Mol Sci. 2023 Nov 20;24(22):16518. doi: 10.3390/ijms242216518. Int J Mol Sci. 2023. PMID: 38003708 Free PMC article. Review. - Effects of Transient Exposure to High Shear on Neutrophil Rolling Behavior.
Lewis CS, Alsmadi NZ, Snyder TA, Schmidtke DW. Lewis CS, et al. Cell Mol Bioeng. 2018 Aug;11(4):279-290. doi: 10.1007/s12195-018-0533-z. Epub 2018 Jun 1. Cell Mol Bioeng. 2018. PMID: 31372187 Free PMC article. - Anti-metastatic functions of type 1 interferons: Foundation for the adjuvant therapy of cancer.
Ortiz A, Fuchs SY. Ortiz A, et al. Cytokine. 2017 Jan;89:4-11. doi: 10.1016/j.cyto.2016.01.010. Epub 2016 Jan 25. Cytokine. 2017. PMID: 26822709 Free PMC article. Review. - The consequence of biologic graft processing on blood interface biocompatibility and mechanics.
Van de Walle AB, Uzarski JS, McFetridge PS. Van de Walle AB, et al. Cardiovasc Eng Technol. 2015 Sep;6(3):303-13. doi: 10.1007/s13239-015-0221-2. Cardiovasc Eng Technol. 2015. PMID: 26322140 Free PMC article. - Stress transmission within the cell.
Stamenović D, Wang N. Stamenović D, et al. Compr Physiol. 2011 Jan;1(1):499-524. doi: 10.1002/cphy.c100019. Compr Physiol. 2011. PMID: 23737186 Free PMC article. Review.
References
- Sugihara-Seki M., Schmid-Schönbein G.W. The fluid shear stress distribution on the membrane of leukocytes in the microcirculation. J. Biomech. Eng. 2003;125:628–638. - PubMed
- Shen Z., Lipowsky H.H. Image enhancement of the in vivo leukocyte-endothelium contact zone using optical sectioning microscopy. Ann. Biomed. Eng. 1997;25:521–535. - PubMed
- Ohashi K.L., Tung D.K.-L., Wilson J., Zweifach B.W., Schmid-Schönbein G.W. Transvascular and interstitial migration of neutrophils in rat mesentery. Microcirculation. 1996;3:199–210. - PubMed
- McIntire L.V., Dewitz T.S., Martin R.R. Mechanical trauma effects on leukocytes. Trans. Am. Soc. Artif. Intern. Organs. 1976;22:444–449. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources