Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system - PubMed (original) (raw)
Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system
Wendy S Garrett et al. Cell. 2007.
Abstract
Inflammatory bowel disease (IBD) has been attributed to overexuberant host immunity or the emergence of harmful intestinal flora. The transcription factor T-bet orchestrates inflammatory genetic programs in both adaptive and innate immunity. We describe a profound and unexpected function for T-bet in influencing the behavior of host inflammatory activity and commensal bacteria. T-bet deficiency in the innate immune system results in spontaneous and communicable ulcerative colitis in the absence of adaptive immunity and increased susceptibility to colitis in immunologically intact hosts. T-bet controls the response of the mucosal immune system to commensal bacteria by regulating TNF-alpha production in colonic dendritic cells, critical for colonic epithelial barrier maintenance. Loss of T-bet influences bacterial populations to become colitogenic, and this colitis is communicable to genetically intact hosts. These findings reveal a novel function for T-bet as a peacekeeper of host-commensal relationships and provide new perspectives on the pathophysiology of IBD.
Figures
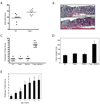
Figure 1. T-bet expression protects against colitis and T-bet-/-x RAG2-/- (TRUC) mice develop spontaneous colitis
(A) WT and T-bet-/- mice, age 8 wks, treated with 4% DSS for 8 days. One representative exp of 3, n=8 per group, p = .0031. (B) Photomicrographs of DSS treated WT (upper panel) and T-bet-/- (lower panel) colons. (C) TRUC mice develop a spontaneous, highly penetrant colitis. Colitis score for each mouse denoted by a dot, the mean for each group shown as a horizontal bar. n=7 per group, all 12 wks old. (D) Mean colon weights (mg) for the mice in 1C. (E) TRUC colitis severity increases with time. Mean colitis score ± stdev, n=10 per group except for 1 week old group, n=8.
Figure 2. Spontaneous colitis in TRUC mice phenocopies human UC and is characterized by a colonic epithelial barrier breach
(A)(1) TRUC mouse (8 wks) with anorectal prolapse. (2) Photograph distal colons RAG2-/- 4 wks (a) 8 wks (c) and TRUC 4 wks (b) 8 wks (d) (anorectal junction at bottom). Vertical bar delineates the inflamed, thickened colonic wall. Colonic wall thickening with prolapsed rectal mucosa in 8 wk TRUC. (3) Normal colonic mucosa 6 wk RAG2-/- mouse, 100x. (4) Representative disease 6 wk TRUC. Note mucosal thickening, surface ulceration, crypt distortion and hyperplasia, and dense mixed inflammatory cell infiltrate in the lamina propria (compare to panel 3), 100x. (B)Intra-rectal FITC-dextran was administered to 3.5 wk T-bet-/-, RAG2-/-, and TRUC. Serum fluorescence measured at the indicated time points. One representative exp of 3, n=4–6 per group, [p =.0002, 60 min TRUC vs RAG2-/-]. (C) Intra-rectal FITC-dextran administered to TRUC at 4, 5, and 6 wks. Serum fluorescence at the indicated time points, [p=.0036, 60 min 6 vs 5 wks]. One representative exp of 3, n= 5 per group. (D) TRUC colonic tight junctions surveyed by EM: representative images prior to 2 wks (1), 3 wks (2), and 4 wks (3), 25,000x. (E) Colonic epithelial discontinuities present in 3.5 wk TRUC. Representative EM images, (1) 800x (2) 1500x (3) 1000x (4) 3000x. (F) Increased apoptosis in TRUC colonic epithelium. WT, T-bet-/-, RAG2-/-, and TRUC colonic epithelium at 5 wks stained with DAPI (blue) and TUNEL (green), 200x. (G) Epithelial crypt and TUNEL+ cell counts. 5 slides generated from each genotype group (2–3 mice per genotype). 500 crypts per genotype were scored.
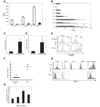
Figure 3. TNF-α drives tissue injury in TRUC colitis and TNF-α overproduction maps to colonic DCs
(A) TNF-α ELISAs on colon explant cultures from 4, 8, and 12 wk old TRUC (open) and RAG2-/- (shaded). One representative exp of 3 is shown, samples pooled, n=4 per group. (B) IL-23, IL-13, IL-12p40, IL-10, IL-6, IL-1α, IL-1β, and IFNγ levels of colon explant cultures from TRUC (open) and RAG2-/- (shaded), samples pooled, n= 4 per group. Data represent the mean of 3 independent exp, error bars denote stdev. (C) 4 wk TRUC were treated with anti-TNF-α Ab or an isotype control for 4 wks. Colitis scores for treated vs control, p=.0023. One representative exp of 3 is shown, n=4 per group. (D) Apoptotic (TUNEL+) epithelial cells counts; anti-TNF-α vs control, p= .0016. (E) TUNEL staining of cultured TRUC colonic epithelial cells (CD45- cytokeratin 5/8+) treated with TNF-α compared to TNF-α treated RAG2-/- colonic epithelial cells (53.8% vs 13.7%). One representative exp of 4 is shown. (F) Colitis scores for 8 wk TRUC TNFR1/p55-/- and TRUC, p<.0001. Colonic cell suspensions were, stained with Abs directed against cell surface markers, and permeabilized to allow for detection of intracytoplasmic TNF-α by flow cytometry. (G) TNF-α production by immunocytes (CD45+). RAG2-/- cells as controls. Abs against Gr-1, F4/80, and CD11c paired with class II were used to identify the immunocytes with the highest TNF-α production. (H) Colonic DC production of TNF-α during the disease course, 2–8 wks. The mean and stdev of 3 independent exp are plotted. Vertical bars denote the right-sided tail of the isotype control staining. Data are representative of 3 independent exp; samples were pooled, n=10–20 per group.
Figure 4. T-bet regulates production of TNF-α in DCs
T-bet is expressed in colonic DCs. (1) CD11c staining RAG2-/- colonic mucosa, 200x. (2) Serial section of (1) stained with anti-T-bet Ab, 4B10, 200x. (3) Sorted colonic, mouse DC stained with anti-T-bet Ab (red) and DAPI (blue), 1000x. (4) Human colonic DC, S100 (pink), anti-T-bet (brown), 1000x. (B) Loss of T-bet expression in BMDCs results in increased production of TNF-α. T-bet binds the TNF-α promoter. (C) qPCR of mouse bone BMDCs ChIP samples. Primer sets A and B, approximately 500 and 1200 bps upstream of the TSS. A schema of the promoter showing T-box consensus sites and the location of primers used. Data are the mean of 3 exp (D) qPCR of human myeloid DC ChIP samples, one representative exp of 3. (E) T-bet negatively regulates TNF-α gene transcription. RAW cells transiently cotransfected with a 3kb TNF-α promoter luciferase construct, a constitutively active Iκκβ mutant construct to activate TNF-α transcription, a Renilla construct for normalization of transfection efficiency, and varying concentrations of a full length T-bet cDNA construct or control plasmid. TNF-α promoter activity is shown as a function of relative luciferase activity. Data are representative of 3 independent exp.
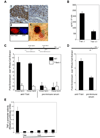
Figure 5. CD4+CD25+ T-regulatory cells control TRUC
(A) 4 week TRUC injected with PBS, 1×106 B cells, or 1×106 naïve T cells, WT or T-bet-/-. Colitis scores at 2 wks post-injection. Representative data from one of 3 exp, n=4 per group. (B). 4 week TRUC mice injected with 75,000 T-regs (CD4+CD25+CD62L+), WT or T-bet-/-, or PBS. Colitis scores at 4 wks post-injection. One representative exp of 3 is shown, n=6–8 per group. (C) Photomicrograph of TRUC colon (T-reg treated). T-regs (CD3, green) and DCs (CD11c, red), 400x. (D) Adoptive transfer of T-regs normalizes TRUC TNF-α levels. 4 week TRUC injected with 75,000 T-regs (CD4+CD25+CD62L+) or PBS. TNF-α levels from colon explant cultures 4 wks post-injection, RAG2-/- colon explants as a control. Samples pooled, n=4–5 per group.
Figure 6. The TRUC niche generates a colitogenic microbial community which is transmissible to T-bet sufficient mice
(A) Colitis scores for 6 wk TRUC treated with vancomycin (V), metronidazole (M), neomycin (N), and ampicillin (A) or metronidazole (M) alone for 6 wks. One representative exp of 3, n=4 per group. (B) Colitis scores for adult progeny of antibiotic-treated mice. Three separate litters are shown for antibiotic treated breeders and 1 litter is shown from untreated breeders, p<.0001. Communicability of TRUC colitis. Colitis scores for cross-fostered RAG2-/- mice (C) and WT mice (D). Mice are from 3 exp (TRUC and RAG2-/-) and 2 exp (TRUC and WT). RAG2-/- reared by RAG2-/- female and WT reared by WT female shown for comparison. (E) TNF-a ELISA on colon explant cultures: RAG2-/- cross-fostered by TRUC, TRUC reared by TRUC, RAG2-/- reared by RAG2-/-, TRUC TNFR1-/- fostered by TRUC, and TRUC TNFR1-/- reared by TRUC TNFR1-/-. Samples were pooled, n=4–8 per group. (F) Colitis scores for RAG2-/- and WT co-housed with TRUC for 8 wks. RAG2-/- and WT co-housed with genotype-identical mice shown for comparison. Horizontal bars represent the mean.
Comment in
- Loss of T-bet sends host-microbe mutualism awry.
Ma A. Ma A. Cell. 2007 Oct 5;131(1):15-7. doi: 10.1016/j.cell.2007.09.030. Cell. 2007. PMID: 17923080
References
- Ansel KM, Greenwald RJ, Agarwal S, Bassing CH, Monticelli S, Interlandi J, Djuretic IM, Lee DU, Sharpe AH, Alt FW, Rao A. Deletion of a conserved Il4 silencer impairs T helper type 1-mediated immunity. Nature Immunology. 2004;5:1251–1259. - PubMed
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
- Baumgart DC, Olivier WA, Reya T, Peritt D, Rombeau JL, Carding S. Mechanims of intestinal epithelial injury and colitis in IL-2 deficient mice. Cell Immunol. 1998;187:52–66. - PubMed
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. - PubMed
- Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases