Self-assembled lamellar complexes of siRNA with lipidic aminoglycoside derivatives promote efficient siRNA delivery and interference - PubMed (original) (raw)
Self-assembled lamellar complexes of siRNA with lipidic aminoglycoside derivatives promote efficient siRNA delivery and interference
Léa Desigaux et al. Proc Natl Acad Sci U S A. 2007.
Abstract
RNA interference requires efficient delivery of small double-stranded RNA molecules into the target cells and their subsequent incorporation into RNA-induced silencing complexes. Although current cationic lipids commonly used for DNA transfection have also been used for siRNA transfection, a clear need still exists for better siRNA delivery to improve the gene silencing efficiency. We synthesized a series of cationic lipids characterized by head groups bearing various aminoglycosides for specific interaction with RNA. siRNA complexation with such lipidic aminoglycoside derivatives exhibited three lipid/siRNA ratio-dependent domains of colloidal stability. Fluorescence and dynamic light-scattering experiments showed that cationic lipid/siRNA complexes were formed at lower charge ratios, exhibited a reduced zone of colloidal instability, and had smaller mean diameters compared with our previously described guanidinium-based cationic lipids. Cryo-transmission electron microscopy and x-ray-scattering experiments showed that, although the final in toto morphology of the lipid/siRNA complexes depended on the aminoglycoside type, there was a general supramolecular arrangement consisting of ordered lamellar domains with an even spacing of 67 A. The most active cationic lipid/siRNA complexes for gene silencing were obtained with 4,5-disubstituted 2-deoxystreptamine aminoglycoside derivatives and were characterized by the siRNA being entrapped in small particles exhibiting lamellar microdomains corresponding to siRNA molecules sandwiched between the lipid bilayers. These results clearly show that lipidic aminoglycoside derivatives constitute a versatile class of siRNA nanocarriers allowing efficient gene silencing.
Conflict of interest statement
Conflict of interest statement: P.L., J.-M.L., and B.P. own stock in In-Cell-Art Co., which commercializes lipidic aminoglycoside derivatives.
Figures
Fig. 1.
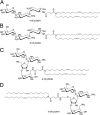
Structure of the lipidic aminoglycoside derivatives. (A) DOST. (B) DOSK. (C) DOSP. (D) DOSN.
Fig. 2.
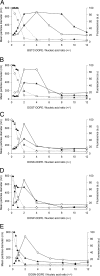
Colloidal stability of cationic lipid/nucleic acid complexes as a function of their charge ratio. Complexes were obtained by mixing BGTC (A), DOST (B), DOSK (C), DOSP (D), and DOSN (E) cationic liposomes at the required concentrations with plasmid DNA (○) or siRNA (▴) at 10 μg/ml. Dynamic light-scattering analysis (solid lines) was performed to assess colloidal stability of the complexes. Ethidium bromide fluorescence measurements (dashed lines) allowed the evaluation of nucleic acid entrapment within complexes. a.u., arbitrary units. Size determination and fluorescence measurements were performed after 1 h of complexation. An arbitrary value of 700 nm was attributed to complexes that were colloidally unstable.
Fig. 3.
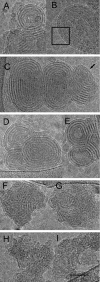
Cryo-TEM micrographs of cationic lipid/siRNA complexes. (A and B) Concentric “onion-like” structure of representative BGTC/siRNA complexes at high magnification. Box in B outlines the regular arrangement of the siRNA molecules between two lipid bilayers. (C) Structure of DOST/siRNA complexes at high magnification. Note the regular arrangement of the RNA molecules at the edge (black arrow). (D and E) Structure of two DOSK/siRNA complexes at high magnification. (F and G) Structure of two DOSP/siRNA complexes at high magnification. (H and I) Structure of two DOSN/siRNA complexes at high magnification. (Scale bar: 50 nm.)
Fig. 4.
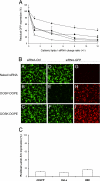
GFP silencing activity of the siRNA complexes formed by various aminoglycoside derivatives. (A) Residual GFP fluorescence after in vitro transfection of d2GFP cells with lipid/siRNA complexes characterized by various +/− charge ratios. GFP-expressing d2GFP cells were transfected with 400 ng of anti-GFP siRNA complexed with liposomes composed of BGTC (●), DOSK (▾), DOST (○), DOSP (■), and DOSN (▿). Residual GFP expression was expressed as the ratio (%) of GFP fluorescence in cells transfected with anti-GFP siRNA to GFP fluorescence in cells transfected with control (non-GFP targeting) siRNA. (B) Fluorescence microscopy visualization of GFP silencing and siRNA internalization. The GFP-expressing d2GFP cells were transfected with control siRNA (A–C in panel B) or 3′-rhodamine-labeled anti-GFP siRNA (D–I in panel B). The siRNA molecules (500 ng per well) were formulated in the absence (“naked” siRNA in A, D, and G in panel B) or in the presence of the cationic lipids DOSP (B, E, and H in panel B), or DOSK (C, F, and I in panel B). The transfected d2GFP cells were observed by using a FITC filter to visualize GFP fluorescence (A–F in panel B) or a rhodamine filter to visualize siRNA internalization (G–I in panel B). (C) Real-time quantitative RT-PCR analysis of human lamin A/C mRNA after transfection of various human cell lines (HEK293, HeLa, and d2GFP cells) with DOSP/siRNA lipoplexes [normalization to hypoxanthine–guanine phosphoribosyltransferase (HPRT1)]. Values are relative to cells transfected under the same experimental condition with a control siRNA.
Similar articles
- Self-assembling complexes between binary mixtures of lipids with different linkers and nucleic acids promote universal mRNA, DNA and siRNA delivery.
Colombani T, Peuziat P, Dallet L, Haudebourg T, Mével M, Berchel M, Lambert O, Habrant D, Pitard B. Colombani T, et al. J Control Release. 2017 Mar 10;249:131-142. doi: 10.1016/j.jconrel.2017.01.041. Epub 2017 Feb 1. J Control Release. 2017. PMID: 28159514 - Quantitative silencing of EGFP reporter gene by self-assembled siRNA lipoplexes of LinOS and cholesterol.
Metwally AA, Blagbrough IS, Mantell JM. Metwally AA, et al. Mol Pharm. 2012 Nov 5;9(11):3384-95. doi: 10.1021/mp300435x. Epub 2012 Oct 25. Mol Pharm. 2012. PMID: 23057412 Free PMC article. - siRNA delivery by a transferrin-associated lipid-based vector: a non-viral strategy to mediate gene silencing.
Cardoso AL, Simões S, de Almeida LP, Pelisek J, Culmsee C, Wagner E, Pedroso de Lima MC. Cardoso AL, et al. J Gene Med. 2007 Mar;9(3):170-83. doi: 10.1002/jgm.1006. J Gene Med. 2007. PMID: 17351968 - Physicochemical parameters of non-viral vectors that govern transfection efficiency.
Barteau B, Chèvre R, Letrou-Bonneval E, Labas R, Lambert O, Pitard B. Barteau B, et al. Curr Gene Ther. 2008 Oct;8(5):313-23. doi: 10.2174/156652308786070961. Curr Gene Ther. 2008. PMID: 18855629 Review. - Cationic Dendrimers for siRNA Delivery: An Overview of Methods for In Vitro/In Vivo Characterization.
Laurini E, Aulic S, Marson D, Fermeglia M, Pricl S. Laurini E, et al. Methods Mol Biol. 2021;2282:209-244. doi: 10.1007/978-1-0716-1298-9_14. Methods Mol Biol. 2021. PMID: 33928579 Review.
Cited by
- Lipid-based nanotherapeutics for siRNA delivery.
Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Schroeder A, et al. J Intern Med. 2010 Jan;267(1):9-21. doi: 10.1111/j.1365-2796.2009.02189.x. J Intern Med. 2010. PMID: 20059641 Free PMC article. Review. - Lipid Nanoparticle-mRNA Formulations for Therapeutic Applications.
Wang C, Zhang Y, Dong Y. Wang C, et al. Acc Chem Res. 2021 Dec 7;54(23):4283-4293. doi: 10.1021/acs.accounts.1c00550. Epub 2021 Nov 18. Acc Chem Res. 2021. PMID: 34793124 Free PMC article. - Lipid-mediated DNA and siRNA Transfection Efficiency Depends on Peptide Headgroup.
Zhang XX, Lamanna CM, Kohman RE, McIntosh TJ, Han X, Grinstaff MW. Zhang XX, et al. Soft Matter. 2013 May 5;9(17):10.1039/C3SM27633C. doi: 10.1039/C3SM27633C. Soft Matter. 2013. PMID: 24391676 Free PMC article. - Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis.
Zhang M, Wang X, Han MK, Collins JF, Merlin D. Zhang M, et al. Nanomedicine (Lond). 2017 Aug;12(16):1927-1943. doi: 10.2217/nnm-2017-0196. Epub 2017 Jun 30. Nanomedicine (Lond). 2017. PMID: 28665164 Free PMC article. - Modified Aminoglycosides Bind Nucleic Acids in High-Molecular-Weight Complexes.
Ying L, Zhu H, Fosso MY, Garneau-Tsodikova S, Fredrick K. Ying L, et al. Antibiotics (Basel). 2020 Feb 21;9(2):93. doi: 10.3390/antibiotics9020093. Antibiotics (Basel). 2020. PMID: 32098020 Free PMC article.
References
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Nature. 1998;391:806–811. - PubMed
- Mello CC, Conte D. Nature. 2004;431:338–342. - PubMed
- Hannon GJ, Rossi JJ. Nature. 2004;431:371–378. - PubMed
- Dykxhoorn DM, Palliser D, Lieberman J. Gene Ther. 2006;13:541–552. - PubMed
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Nature. 2001;24:494–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources