Tumor-targeted delivery of siRNA by self-assembled nanoparticles - PubMed (original) (raw)
Tumor-targeted delivery of siRNA by self-assembled nanoparticles
Shyh-Dar Li et al. Mol Ther. 2008 Jan.
Abstract
We have developed a self-assembled nanoparticle (NP) that efficiently delivers small interfering RNA (siRNA) to the tumor by intravenous (IV) administration. The NP was obtained by mixing carrier DNA, siRNA, protamine, and lipids, followed by post-modification with polyethylene glycol and a ligand, anisamide. Four hours after IV injection of the formulation into a xenograft model, 70-80% of injected siRNA/g accumulated in the tumor, approximately 10% was detected in the liver and approximately 20% recovered in the lung. Confocal microscopy showed that fluorescent-labeled siRNA was efficiently delivered into the cytoplasm of the sigma receptor expressing NCI-H460 xenograft tumor by the targeted NPs, whereas free siRNA and non-targeted NPs showed little uptake. Three daily injections (1.2 mg/kg) of siRNA formulated in the targeted NPs silenced the epidermal growth factor receptor (EGFR) in the tumor and induced approximately 15% tumor cell apoptosis. Forty percent tumor growth inhibition was achieved by treatment with targeted NPs, while complete inhibition lasted for 1 week when combined with cisplatin. The serum level of liver enzymes and body weight monitoring during the treatment indicated a low level of toxicity of the formulation. The carrier itself also showed little immunotoxicity (IMT).
Figures
Figure 1. Serum concentration profiles of FAM-siRNA in different formulations
Data = mean ± SD, n = 4–8. NP, nanoparticle; siRNA, small interfering RNA.
Figure 2. Tissue distribution study
(a) Fluorescence signal of FAM-siRNA in different tissues detected by the Xenogen IVIS imaging system. (b) Tissue distribution of FAM-siRNA in different formulations. Data = mean ± SD, n = 3–4. NP, nanoparticle; siRNA, small interfering RNA.
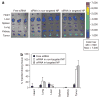
Figure 3. Tumoral uptake of FAM-siRNA in different formulations
Blue arrows indicate the extracellular space and pink arrows indicate the intracellular uptake of FAM-siRNA. Magnification = ×400 (xyz images), ×630 (xzy images). NP, nanoparticle; siRNA, small interfering RNA.
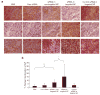
Figure 4. Immunohistochemical analysis of the tumor samples
(a) Immunohistochemical staining on tumor sections: epidermal growth factor receptor (upper), prostate apoptotic response 4 (middle), and apoptosis inducing factor (AIF) (bottom). Magnification = ×200. (b) Quantitative analysis of nuclear translocation of AIF in the tumors treated with different formulations. **indicates P < 0.01, *indicates P < 0.05. NP, nanoparticle; PBS, phosphate-buffered saline; siRNA, small interfering RNA.
Figure 5. Western blot analysis of epidermal growth factor receptor (EGFR) in the NCI-H460 xenograft tumor after treatment with different formulations
NP, nanoparticle; PBS, phosphate-buffered saline; siRNA, small interfering RNA.
Figure 6. NCI-H460 xenograft tumor growth inhibition by small interfering RNA (siRNA) in different formulations with or without the combination of cisplatin
Solid arrows indicate the intravenous administrations of siRNA (1.2 mg/kg) and dash-line arrows indicate the intra-peritoneal injections of cisplatin (3 mg/kg). Data = mean, n = 6–9. SD of the data points is not shown for clarity.
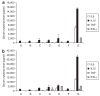
Figure 7. Serum cytokine analysis
Serum cytokine levels in the (a) athymic nude mice and (b) C57BL/6 mice 2 hours after the injection of different formulations at the dose of 1.2 mg siRNA/kg (2.4 mg DNA/kg for group F). A, phosphate-buffered saline (PBS); B, epidermal growth factor receptor (EGFR) small interfering RNA (siRNA) in PBS; C, EGFR siRNA and calf thymus DNA in non-targeted nanoparticle (NP); D, EGFR siRNA and calf thymus DNA in targeted NP; E, MDM2 siRNA and calf thymus DNA in targeted NP; F, calf thymus DNA in targeted NP (empty NP); G, EGFR siRNA and plasmid DNA in targeted NP. Data = mean ± SD, n = 3–8.
Similar articles
- Systemic delivery of siRNA via LCP nanoparticle efficiently inhibits lung metastasis.
Yang Y, Li J, Liu F, Huang L. Yang Y, et al. Mol Ther. 2012 Mar;20(3):609-15. doi: 10.1038/mt.2011.270. Epub 2011 Dec 20. Mol Ther. 2012. PMID: 22186791 Free PMC article. - Novel cationic lipid that delivers siRNA and enhances therapeutic effect in lung cancer cells.
Chen Y, Sen J, Bathula SR, Yang Q, Fittipaldi R, Huang L. Chen Y, et al. Mol Pharm. 2009 May-Jun;6(3):696-705. doi: 10.1021/mp800136v. Mol Pharm. 2009. PMID: 19267451 Free PMC article. - Efficient gene silencing in metastatic tumor by siRNA formulated in surface-modified nanoparticles.
Li SD, Chono S, Huang L. Li SD, et al. J Control Release. 2008 Feb 18;126(1):77-84. doi: 10.1016/j.jconrel.2007.11.002. Epub 2007 Nov 17. J Control Release. 2008. PMID: 18083264 Free PMC article. - Systemic delivery of siRNA by T7 peptide modified core-shell nanoparticles for targeted therapy of breast cancer.
Yu MZ, Pang WH, Yang T, Wang JC, Wei L, Qiu C, Wu YF, Liu WZ, Wei W, Guo XY, Zhang Q. Yu MZ, et al. Eur J Pharm Sci. 2016 Sep 20;92:39-48. doi: 10.1016/j.ejps.2016.06.020. Epub 2016 Jun 26. Eur J Pharm Sci. 2016. PMID: 27355138 - An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor.
Chono S, Li SD, Conwell CC, Huang L. Chono S, et al. J Control Release. 2008 Oct 6;131(1):64-9. doi: 10.1016/j.jconrel.2008.07.006. Epub 2008 Jul 13. J Control Release. 2008. PMID: 18674578 Free PMC article.
Cited by
- Pharmacokinetic Behaviors of Intravenously Administered siRNA in Glandular Tissues.
Huang Y, Cheng Q, Ji JL, Zheng S, Du L, Meng L, Wu Y, Zhao D, Wang X, Lai L, Cao H, Xiao K, Gao S, Liang Z. Huang Y, et al. Theranostics. 2016 Jun 18;6(10):1528-41. doi: 10.7150/thno.15246. eCollection 2016. Theranostics. 2016. PMID: 27446488 Free PMC article. - Poly-L-arginine and dextran sulfate-based nanocomplex for epidermal growth factor receptor (EGFR) siRNA delivery: its application for head and neck cancer treatment.
Cho HJ, Chong S, Chung SJ, Shim CK, Kim DD. Cho HJ, et al. Pharm Res. 2012 Apr;29(4):1007-19. doi: 10.1007/s11095-011-0642-z. Epub 2011 Dec 15. Pharm Res. 2012. PMID: 22169985 - Targeted delivery of EV peptide to tumor cell cytoplasm using lipid coated calcium carbonate nanoparticles.
Kim SK, Foote MB, Huang L. Kim SK, et al. Cancer Lett. 2013 Jul 1;334(2):311-8. doi: 10.1016/j.canlet.2012.07.011. Epub 2012 Jul 14. Cancer Lett. 2013. PMID: 22796364 Free PMC article. - Lipopolyplex for Therapeutic Gene Delivery and Its Application for the Treatment of Parkinson's Disease.
Chen W, Li H, Liu Z, Yuan W. Chen W, et al. Front Aging Neurosci. 2016 Apr 5;8:68. doi: 10.3389/fnagi.2016.00068. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27092073 Free PMC article. Review.
References
- Jana S, Chakraborty C, Nandi S, Deb JK. RNA interference: potential therapeutic targets. Appl Microbiol Biotechnol. 2004;65:649–657. - PubMed
- Li SD, Huang L. Gene therapy progress and prospects: non-viral gene therapy by systemic delivery. Gene Ther. 2006;13:1313–1319. - PubMed
- Li W, Szoka FC., Jr Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24:438–449. - PubMed
- Grzelinski M, Urban-Klein B, Martens T, Lamszus K, Bakowsky U, Hobel S, et al. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum Gene Ther. 2006;17:751–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA129835/CA/NCI NIH HHS/United States
- R01 CA129835-01A1/CA/NCI NIH HHS/United States
- R56 AI048851/AI/NIAID NIH HHS/United States
- R56 AI048851-07A1/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous