Bilateral activity-dependent interactions in the developing corticospinal system - PubMed (original) (raw)
Comparative Study
Bilateral activity-dependent interactions in the developing corticospinal system
Kathleen M Friel et al. J Neurosci. 2007.
Abstract
Activity-dependent competition between the corticospinal (CS) systems in each hemisphere drives postnatal development of motor skills and stable CS tract connections with contralateral spinal motor circuits. Unilateral restriction of motor cortex (M1) activity during an early postnatal critical period impairs contralateral visually guided movements later in development and in maturity. Silenced M1 develops aberrant connections with the contralateral spinal cord whereas the initially active M1, in the other hemisphere, develops bilateral connections. In this study, we determined whether the aberrant pattern of CS tract terminations and motor impairments produced by early postnatal M1 activity restriction could be abrogated by reducing activity-dependent synaptic competition from the initially active M1 later in development. We first inactivated M1 unilaterally between postnatal weeks 5-7. We next inactivated M1 on the other side from weeks 7-11 (alternate inactivation), to reduce the competitive advantage that this side may have over the initially inactivated side. Alternate inactivation redirected aberrant contralateral CS tract terminations from the initially silenced M1 to their normal spinal territories and reduced the density of aberrant ipsilateral terminations from the initially active side. Normal movement endpoint control during visually guided locomotion was fully restored. This reorganization of CS terminals reveals an unsuspected late plasticity after the critical period for establishing the pattern of CS terminations in the spinal cord. Our findings show that robust bilateral interactions between the developing CS systems on each side are important for achieving balance between contralateral and ipsilateral CS tract connections and visuomotor control.
Figures
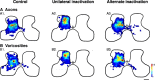
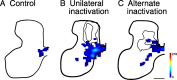
Figure 1.
Alternate inactivation promotes redistribution of CS terminations to intermediate and ventral motor laminas after previous M1 inactivation. Color-coded average density maps are shown for age-matched controls (left column; n = 4), animals with unilateral inactivation (center; n = 4), and animals with alternate inactivation (right; n = 6). Axon density (terminals and preterminal axons) maps for are shown in A and bouton density maps in B. The contours in A graph the densest regions (yellow; maximal 40%) from the individual animals comprising the averages. Scale bar, 500 μm; color scale: A, 0–216 μm axon/μm2 area; B, 0–3.5 × 10−2 boutons/μm.
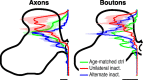
Figure 2.
Unilateral inactivation (inact.) shifts the distribution of CS terminations dorsally in the gray matter and alternate inactivation restores terminations to the intermediate and ventral laminas. Dorsoventral distributions of axon (left) and bouton (right) density from the dorsal to ventral margins of the contralateral gray mater are shown. Solid lines plot the mean pixel values at each normalized depth; light shading plots ±SEM. Data from controls (ctrl.) are shown in green, unilateral inactivation in red, and alternate inactivation in blue. To normalize for differences in the size of the gray matter across animals, all graphs were interpolated to 1000 points between the dorsal and ventral margins of the gray matter. Scale bars: axons, 500 μm axon/μm2 area; varicosities, 5 × 10−3 boutons/μm.
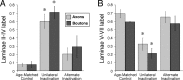
Figure 3.
A, B, The proportion of axonal labeling (light gray) and boutons (dark gray) within laminas 2–4 (A) and laminas 5–7 (B) after alternate inactivation are similar to controls. Data from controls, unilateral inactivation, and alternate inactivation are shown. Asterisks show significant differences for axon labeling and bouton density between the unilateral inactivation group and either the controls (p < 0.01) or the alternate inactivation (p < 0.01) groups. Error bars indicate SEM.
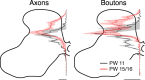
Figure 4.
CS terminations have similar distribution immediately after the alternate inactivation (PW 11) and 4 weeks later (PW 15). Dorsoventral distributions of axon (left) and bouton (right) density from the dorsal to ventral margins of the contralateral gray mater are shown for animals examined at PW 11 (n = 2; black) and PW 15/16 (n = 4; red). Solid lines and shading are similar to Figure 2. Scale bars: axons, 500 μm axon/μm2 area; varicosities, 5 × 10−3 boutons/μm.
Figure 5.
Alternate inactivation reduces ipsilateral CS tract terminations. A–C, Averaged regional distribution of ipsilateral CS axon terminations for controls (n = 4; A, replotted from Fig. 1_A_), animals receiving unilateral inactivation only (B; n = 3) and alternate inactivation (C; n = 3). Conventions are similar to those in Figure 1_A_. We used either LY (n = 2) or BDA (n = 1) to anterogradely label projections from the initially active (right) side, depending on which tracer we did not use for the initially inactive side. The contours in B and C plot the region of maximal contralateral label (inner; 40%; yellow) and an estimate of total contralateral label (outer; 80%; light blue). All maps are plotted using the same color scale. Scale bar, 500 μm.
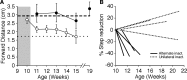
Figure 6.
Alternate inactivation restores end point control during visually guided locomotion. A, Forward distance of the forepaw contralateral to the first inactivation by week of representative cats after unilateral (filled circles) or alternate inactivation (open circles). Forward distance remains higher than controls (dotted line, average, n = 4) even 2 months after unilateral inactivation. The dashed line represents the average forward distance for unilaterally inactivated cats (n = 5). After alternate inactivation, forward distance returns to control levels 1 month after the second inactivation. Average control forward distance is plotted as a dotted line and unilateral inactivation as a dashed line. B, Plot showing the lack of recovery in animals with unilateral inactivation (inact.) and recovery after alternate inactivation. Unilateral inactivation resulted in persistent overstepping (dotted lines; n = 4; data from a previous study for animals tested longer than 16 weeks) (Friel et al., 2007). After alternate inactivation (solid lines), all animals recovered in <15 weeks.
Similar articles
- Pyramidal tract stimulation restores normal corticospinal tract connections and visuomotor skill after early postnatal motor cortex activity blockade.
Salimi I, Friel KM, Martin JH. Salimi I, et al. J Neurosci. 2008 Jul 16;28(29):7426-34. doi: 10.1523/JNEUROSCI.1078-08.2008. J Neurosci. 2008. PMID: 18632946 Free PMC article. - Motor Cortex Activity Organizes the Developing Rubrospinal System.
Williams PT, Martin JH. Williams PT, et al. J Neurosci. 2015 Sep 30;35(39):13363-74. doi: 10.1523/JNEUROSCI.1719-15.2015. J Neurosci. 2015. PMID: 26424884 Free PMC article. - Activity-dependent plasticity improves M1 motor representation and corticospinal tract connectivity.
Chakrabarty S, Friel KM, Martin JH. Chakrabarty S, et al. J Neurophysiol. 2009 Mar;101(3):1283-93. doi: 10.1152/jn.91026.2008. Epub 2008 Dec 17. J Neurophysiol. 2009. PMID: 19091920 Free PMC article. - Activity- and use-dependent plasticity of the developing corticospinal system.
Martin JH, Friel KM, Salimi I, Chakrabarty S. Martin JH, et al. Neurosci Biobehav Rev. 2007;31(8):1125-35. doi: 10.1016/j.neubiorev.2007.04.017. Epub 2007 May 17. Neurosci Biobehav Rev. 2007. PMID: 17599407 Free PMC article. Review. - The corticospinal system: from development to motor control.
Martin JH. Martin JH. Neuroscientist. 2005 Apr;11(2):161-73. doi: 10.1177/1073858404270843. Neuroscientist. 2005. PMID: 15746384 Review.
Cited by
- Chronic activation of corticospinal tract neurons after pyramidotomy injury enhances neither behavioral recovery nor axonal sprouting.
Wang Z, Brannigan M, Friedrich L, Blackmore MG. Wang Z, et al. bioRxiv [Preprint]. 2024 Oct 26:2024.10.25.620314. doi: 10.1101/2024.10.25.620314. bioRxiv. 2024. PMID: 39484429 Free PMC article. Preprint. - Physical Exercise Keeps the Brain Connected: Biking Increases White Matter Integrity in Patients With Schizophrenia and Healthy Controls.
Svatkova A, Mandl RC, Scheewe TW, Cahn W, Kahn RS, Hulshoff Pol HE. Svatkova A, et al. Schizophr Bull. 2015 Jul;41(4):869-78. doi: 10.1093/schbul/sbv033. Epub 2015 Mar 31. Schizophr Bull. 2015. PMID: 25829377 Free PMC article. Clinical Trial. - Motor Experience Reprograms Development of a Genetically-Altered Bilateral Corticospinal Motor Circuit.
Serradj N, Martin JH. Serradj N, et al. PLoS One. 2016 Sep 27;11(9):e0163775. doi: 10.1371/journal.pone.0163775. eCollection 2016. PLoS One. 2016. PMID: 27673329 Free PMC article. - Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth.
Carmel JB, Berrol LJ, Brus-Ramer M, Martin JH. Carmel JB, et al. J Neurosci. 2010 Aug 11;30(32):10918-26. doi: 10.1523/JNEUROSCI.1435-10.2010. J Neurosci. 2010. PMID: 20702720 Free PMC article. - The cerebellum, sensitive periods, and autism.
Wang SS, Kloth AD, Badura A. Wang SS, et al. Neuron. 2014 Aug 6;83(3):518-32. doi: 10.1016/j.neuron.2014.07.016. Neuron. 2014. PMID: 25102558 Free PMC article. Review.
References
- Alisky JM, Swink TD, Tolbert DL. The postnatal spatial and temporal development of corticospinal projections in cats. Exp Brain Res. 1992;88:265–276. - PubMed
- Bouza H, Rutherford M, Acolet D, Pennock JM, Dubowitz LM. Evolution of early hemiplegic signs in full-term infants with unilateral brain lesions in the neonatal period: a prospective study. Neuropediatrics. 1994;25:201–207. - PubMed
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–1247. - PubMed
- Chakrabarty S, Martin JH. Postnatal development of the motor representation in primary motor cortex. J Neurophysiol. 2000;84:2582–2594. - PubMed
- Chakrabarty S, Friel K, Martin JH. Changes in M1 motor maps reflect plasticity of spinal terminations. Soc Neurosci Abstr. 2007;33:132–24.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources