The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation - PubMed (original) (raw)
The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation
Antje M Wengner et al. Blood. 2008.
Abstract
In this study, we have identified a unique combinatorial effect of the chemokines KC/MIP-2 and the cytokine granulocyte colony-stimulating factor (G-CSF) with respect to the rapid mobilization of neutrophils from the bone marrow in a model of acute peritonitis. At 2 hours following an intraperitoneal injection of thioglycollate, there was a 4.5-fold increase in blood neutrophil numbers, which was inhibited 84% and 72% by prior administration of blocking mAbs against either the chemokines KC/MIP-2 or G-CSF, respectively. An intraperitoneal injection of G-CSF acted remotely to stimulate neutrophil mobilization, but did not elicit recruitment into the peritoneum. Further, in vitro G-CSF was neither chemotactic nor chemokinetic for murine neutrophils, and had no priming effect on chemotaxis stimulated by chemokines. Here, we show that, in vitro and in vivo, G-CSF induces neutrophil mobilization by disrupting their SDF-1alpha-mediated retention in the bone marrow. Using an in situ perfusion system of the mouse femoral bone marrow to directly assess mobilization, KC and G-CSF mobilized 6.8 x 10(6) and 5.4 x 10(6) neutrophils, respectively, while the infusion of KC and G-CSF together mobilized 19.5 x 10(6) neutrophils, indicating that these factors act cooperatively with respect to neutrophil mobilization.
Figures
Figure 1
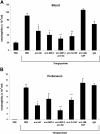
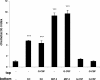
KC/MIP-2 and G-CSF but not GM-CSF contribute to blood and tissue neutrophilia in TG-induced peritonitis. Neutralizing antibodies against KC (50 μg), MIP-2 (20 μg), KC and MIP-2, G-CSF (150 μg), GM-CSF (50 μg), or rat isotype-control mAb (mixture of control isotype IgGs) were administered intraperitoneally 20 minutes before intraperitoneal injection of 3% thioglycollate (TG). The number of neutrophils per milliliter of blood (A) and the number of neutrophils per milliliter of peritoneal lavage fluid (B) were determined 2 hours following TG injection. Results are expressed as means plus or minus SEM. Data from 6 independent experiments are combined (n = 4-15 mice). **P < .01 (1-way ANOVA and Dunnett test).
Figure 2
Intravenous administration of KC, MIP-2, and G-CSF stimulates the mobilization of neutrophils from the bone marrow into the blood. Mice injected intravenously with either 100 μL of PBS, KC, MIP-2, or G-CSF (660 nM each). The number of neutrophils per milliliter of blood (A) and the number of bone marrow neutrophils (per femur) (B) were determined 2 hours following injection. Results are expressed as means plus or minus SEM (n = 4 mice). *P < .05; **P < .01 (1-way ANOVA and Dunnett test).
Figure 3
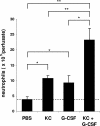
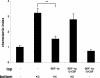
Mobilization of neutrophils from the perfused femoral bone marrow in mice following the infusion of KC and G-CSF alone or in combination. The number of neutrophils (Gr-1high) mobilized from the bone marrow after infusion of PBS, G-CSF (15 nM), KC (15 nM), or G-CSF and KC are shown. Results are expressed as means plus or minus SEM (n = 4 mice). *P < .05; **P < .01 (1-way ANOVA and Dunnett test).
Figure 4
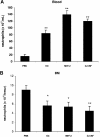
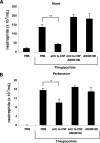
G-CSF and MIP-2 both mobilize neutrophils from the bone marrow, while MIP-2 but not G-CSF stimulates recruitment into tissue. Mice were injected intraperitoneally with either 200 μL of PBS, MIP-2, or G-CSF (330 nM each). The number of neutrophils per milliliter of blood (A) and the number of neutrophils per milliliter of peritoneal lavage fluid (B) were determined 2 hours following injection. Results are expressed as means plus or minus SEM (n = 3 mice). *P < .05; **P < .01 (1-way ANOVA and Dunnett test).
Figure 5
G-CSF does not stimulate the chemotaxis or chemokinesis of murine neutrophils. Freshly isolated murine bone marrow neutrophils were used in a chemotaxis assay. Neutrophils were placed on top of a chemotaxis plate in the presence or absence of G-CSF (10 nM). KC (1 nM), MIP-2 (1 nM), or G-CSF (10 nM) were added to the bottom chamber as indicated. Migration of neutrophils is expressed as the mean number of cells counted per bottom well, mean plus or minus SD. A representative figure of 3 independent experiments is shown (n = 3). ***P < .001 (1-way ANOVA and Dunnett test).
Figure 6
G-CSF blocks the retention of neutrophils by SDF-1α. Freshly isolated murine bone marrow neutrophils were used in a chemotaxis assay and then placed on top of the chemotaxis plate in the presence or absence of G-CSF (10 nM) as indicated. Neutrophils were preincubated in the presence or absence of SDF-1α (50 nM) for 15 minutes at 37°C. KC (1 nM) was added to the bottom chamber as indicated. Migration of neutrophils is expressed as the mean number of cells counted per bottom well, mean plus or minus SD. A representative figure of 4 independent experiments is shown (n = 3). **P < .01 (1-way ANOVA and Dunnett test).
Figure 7
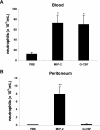
Inhibition of neutrophil mobilization by blockade of G-CSF is abrogated by administration of AMD3100. Anti–G-CSF–blocking antibody (150 μg) and AMD3100 (100 μg) alone or in combination were administered intraperitoneally 20 minutes before intraperitoneal injection of 3% TG. The number of neutrophils per milliliter of blood (A) and the number of neutrophils per milliliter of peritoneal lavage fluid (B) were determined 2 hours following TG injection. Results are expressed as means plus or minus SEM (n = 3). *P < .05; **P < .005 (1-way ANOVA and Dunnett test).
Similar articles
- GRK6 deficiency is associated with enhanced CXCR4-mediated neutrophil chemotaxis in vitro and impaired responsiveness to G-CSF in vivo.
Vroon A, Heijnen CJ, Raatgever R, Touw IP, Ploemacher RE, Premont RT, Kavelaars A. Vroon A, et al. J Leukoc Biol. 2004 Apr;75(4):698-704. doi: 10.1189/jlb.0703320. Epub 2004 Jan 2. J Leukoc Biol. 2004. PMID: 14704365 - CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow.
Eash KJ, Greenbaum AM, Gopalan PK, Link DC. Eash KJ, et al. J Clin Invest. 2010 Jul;120(7):2423-31. doi: 10.1172/JCI41649. J Clin Invest. 2010. PMID: 20516641 Free PMC article. - STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction.
Nguyen-Jackson H, Panopoulos AD, Zhang H, Li HS, Watowich SS. Nguyen-Jackson H, et al. Blood. 2010 Apr 22;115(16):3354-63. doi: 10.1182/blood-2009-08-240317. Epub 2010 Feb 25. Blood. 2010. PMID: 20185584 Free PMC article. - Neutrophil mobilization and clearance in the bone marrow.
Furze RC, Rankin SM. Furze RC, et al. Immunology. 2008 Nov;125(3):281-8. doi: 10.1111/j.1365-2567.2008.02950.x. Immunology. 2008. PMID: 19128361 Free PMC article. Review.
Cited by
- G-CSF maintains controlled neutrophil mobilization during acute inflammation by negatively regulating CXCR2 signaling.
Bajrami B, Zhu H, Kwak HJ, Mondal S, Hou Q, Geng G, Karatepe K, Zhang YC, Nombela-Arrieta C, Park SY, Loison F, Sakai J, Xu Y, Silberstein LE, Luo HR. Bajrami B, et al. J Exp Med. 2016 Sep 19;213(10):1999-2018. doi: 10.1084/jem.20160393. Epub 2016 Aug 22. J Exp Med. 2016. PMID: 27551153 Free PMC article. - Neutrophils restrain allergic airway inflammation by limiting ILC2 function and monocyte-dendritic cell antigen presentation.
Patel DF, Peiró T, Bruno N, Vuononvirta J, Akthar S, Puttur F, Pyle CJ, Suveizdytė K, Walker SA, Singanayagam A, Carlin LM, Gregory LG, Lloyd CM, Snelgrove RJ. Patel DF, et al. Sci Immunol. 2019 Nov 8;4(41):eaax7006. doi: 10.1126/sciimmunol.aax7006. Sci Immunol. 2019. PMID: 31704734 Free PMC article. - Neutrophil kinetics in health and disease.
Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Summers C, et al. Trends Immunol. 2010 Aug;31(8):318-24. doi: 10.1016/j.it.2010.05.006. Trends Immunol. 2010. PMID: 20620114 Free PMC article. Review. - 1-Palmitoyl-2-Linoleoyl-3-Acetyl-rac-Glycerol (PLAG) Rapidly Resolves LPS-Induced Acute Lung Injury Through the Effective Control of Neutrophil Recruitment.
Lee HR, Shin SH, Kim JH, Sohn KY, Yoon SY, Kim JW. Lee HR, et al. Front Immunol. 2019 Sep 18;10:2177. doi: 10.3389/fimmu.2019.02177. eCollection 2019. Front Immunol. 2019. PMID: 31620122 Free PMC article. - Structural Insights Into How Proteoglycans Determine Chemokine-CXCR1/CXCR2 Interactions: Progress and Challenges.
Rajarathnam K, Desai UR. Rajarathnam K, et al. Front Immunol. 2020 Apr 24;11:660. doi: 10.3389/fimmu.2020.00660. eCollection 2020. Front Immunol. 2020. PMID: 32391006 Free PMC article. Review.
References
- Collins PD, Jose PJ, Williams TJ. The sequential generation of neutrophil chemoattractant proteins in acute inflammation in the rabbit in vivo. Relationship between C5a and proteins with the characteristics of IL-8/neutrophil-activating protein 1. J Immunol. 1991;146:677–684. - PubMed
- Jose PJ, Collins PD, Perkins JA, et al. Identification of a second neutrophil-chemoattractant cytokine generated during an inflammatory reaction in the rabbit peritoneal cavity in vivo: purification, partial amino acid sequence and structural relationship to melanoma-growth-stimulatory activity. Biochem J. 1991;278:493–497. - PMC - PubMed
- Ley K. Integration of inflammatory signals by rolling neutrophils. Immunol Rev. 2002;186:8–18. - PubMed
- Ley K. Arrest chemokines. Microcirculation. 2003;10:289–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases