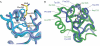Structural and functional analyses of methyl-lysine binding by the malignant brain tumour repeat protein Sex comb on midleg - PubMed (original) (raw)
Structural and functional analyses of methyl-lysine binding by the malignant brain tumour repeat protein Sex comb on midleg
Clemens Grimm et al. EMBO Rep. 2007 Nov.
Abstract
Sex comb on midleg (Scm) is a member of the Polycomb group of proteins involved in the maintenance of repression of Hox and other developmental control genes in Drosophila. The two malignant brain tumour (MBT) repeats of Scm form a domain that preferentially binds to monomethylated lysine residues either as a free amino acid or in the context of peptides, while unmodified or di- or trimethylated lysine residues are bound with significantly lower affinity. The crystal structure of a monomethyl-lysine-containing histone tail peptide bound to the MBT repeat domain shows that the methyl-lysine side chain occupies a binding pocket in the second MBT repeat formed by three conserved aromatic residues and one aspartate. Insertion of the monomethylated side chain into this pocket seems to be the main contributor to the binding affinity. Functional analyses in Drosophila show that the MBT domain of Scm and its methyl-lysine-binding activity are required for repression of Hox genes.
Figures
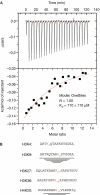
Figure 1
Binding of methyl-lysine peptides to the MBT repeats of Scm. (A) ITC profile for the binding of a 16-mer H4 tail peptide with monomethylated lysine (K) 9 residue to the Scm MBT repeat domain. Data were fitted to a one-site model with stoichiometry of 1:1. The _K_D value is 710 μm (see Table 1). (B) Methyl-lysine peptides used for the ITC experiments as summarized in Table 1. Sequences of mono-methylated H3K9 and H4K20 peptides used for co-crystallization experiments are underlined. ITC, isothermal titration calorimetry; MBT, malignant brain tumour; Scm, Sex comb on midleg.
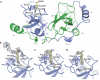
Figure 2
Structure of the Scm MBT repeat domain. (A) Amino- and carboxy-terminal MBT repeats are depicted in green and blue. Methyl-lysine bound to the second MBT repeat is depicted in yellow. (B) Arg(Kme1)Ser peptide bound to the second MBT repeat. The electron density for a 2_F_obs– F_calc-simulated annealing omit map is contoured at 0.7_σ. The well-ordered mono-methyl-lysine moiety is depicted in yellow, and disordered flanking residues are shown in grey. (C) Mono-methyl-lysine-bound structure contoured at 0.7_σ_. (D) Dimethyl-lysine-bound structure. MBT, malignant brain tumour; Scm, Sex comb on midleg.
Figure 3
Ribbon representation of the Scm-binding pocket. (A) View of the binding pocket with superimposed apo (turquoise) and monomethylated lysine-bound structures (light blue). The monomethylated lysine is shown in yellow. (B) Superposition of the amino-terminal (green) with the carboxy-terminal MBT (blue) repeat of Scm. MBT, malignant brain tumour; Scm, Sex comb on midleg.
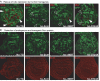
Figure 4
Requirement for the MBT repeat domain of Scm in Hox gene repression. Wing imaginal discs with Scm D1 mutant clones from animals carrying no transgene (no TG) or the indicated hsp70-Scm transgene were used. Discs were stained with antibodies against Ubx (red, top row) or against Scm protein (red, bottom row); Scm D1 mutant clones are marked by the absence of GFP (green, top and middle rows). In the middle and bottom rows, the GFP and Scm protein signals of the same disc are shown separately. Animals were repeatedly heat-shocked for 1 h every 12 h beginning at the time of clone induction, and discs were analysed 96 h after clone induction. (A) In the absence of an hsp70-Scm transgene (no TG), Ubx is strongly misexpressed in most Scm mutant clones in the wing pouch. In animals carrying hsp70-Scm, Ubx stays repressed in all mutant cells. The _hsp70-Scm_ΔMBT and hsp70-Scm Asp324Ala transgenes are unable to fully rescue repression of Ubx, and small clusters of Ubx-positive cells are present in the region of the presumptive wing margin (white arrowheads), but note that Ubx repression is nevertheless rescued in a considerable fraction of clone cells (empty arrowheads) compared with animals lacking a transgene. (B) The transgene-encoded hs-Scm, hs-ScmΔMBT and hs-ScmAsp324Ala proteins are all expressed at comparable levels (bottom row). GFP, green fluorescent protein; hs, heat shock; hsp70, heat-shock protein 70; MBT, malignant brain tumour; Scm, Sex comb on midleg; Ubx, Ultrabithorax.
Similar articles
- Molecular recognition of histone lysine methylation by the Polycomb group repressor dSfmbt.
Grimm C, Matos R, Ly-Hartig N, Steuerwald U, Lindner D, Rybin V, Müller J, Müller CW. Grimm C, et al. EMBO J. 2009 Jul 8;28(13):1965-77. doi: 10.1038/emboj.2009.147. Epub 2009 Jun 4. EMBO J. 2009. PMID: 19494831 Free PMC article. - The malignant brain tumor repeats of human SCML2 bind to peptides containing monomethylated lysine.
Santiveri CM, Lechtenberg BC, Allen MD, Sathyamurthy A, Jaulent AM, Freund SM, Bycroft M. Santiveri CM, et al. J Mol Biol. 2008 Oct 24;382(5):1107-12. doi: 10.1016/j.jmb.2008.07.081. Epub 2008 Aug 3. J Mol Biol. 2008. PMID: 18706910 - Structural studies of a four-MBT repeat protein MBTD1.
Eryilmaz J, Pan P, Amaya MF, Allali-Hassani A, Dong A, Adams-Cioaba MA, Mackenzie F, Vedadi M, Min J. Eryilmaz J, et al. PLoS One. 2009 Oct 20;4(10):e7274. doi: 10.1371/journal.pone.0007274. PLoS One. 2009. PMID: 19841675 Free PMC article. - Chromatin regulation: how complex does it get?
Meier K, Brehm A. Meier K, et al. Epigenetics. 2014 Nov;9(11):1485-95. doi: 10.4161/15592294.2014.971580. Epigenetics. 2014. PMID: 25482055 Free PMC article. Review. - Steps toward understanding the inheritance of repressive methyl-lysine marks in histones.
Reinberg D, Chuikov S, Farnham P, Karachentsev D, Kirmizis A, Kuzmichev A, Margueron R, Nishioka K, Preissner TS, Sarma K, Abate-Shen C, Steward R, Vaquero A. Reinberg D, et al. Cold Spring Harb Symp Quant Biol. 2004;69:171-82. doi: 10.1101/sqb.2004.69.171. Cold Spring Harb Symp Quant Biol. 2004. PMID: 16117647 Review. No abstract available.
Cited by
- Lysine Methylation-Dependent Proteolysis by the Malignant Brain Tumor (MBT) Domain Proteins.
Sun H, Zhang H. Sun H, et al. Int J Mol Sci. 2024 Feb 13;25(4):2248. doi: 10.3390/ijms25042248. Int J Mol Sci. 2024. PMID: 38396925 Free PMC article. Review. - Histone Readers and Their Roles in Cancer.
Wen H, Shi X. Wen H, et al. Cancer Treat Res. 2023;190:245-272. doi: 10.1007/978-3-031-45654-1_8. Cancer Treat Res. 2023. PMID: 38113004 Free PMC article. - Epigenetic Reprogramming of the Glucose Metabolic Pathways by the Chromatin Effectors During Cancer.
Mondal P, Tiwary N, Sengupta A, Dhang S, Roy S, Das C. Mondal P, et al. Subcell Biochem. 2022;100:269-336. doi: 10.1007/978-3-031-07634-3_9. Subcell Biochem. 2022. PMID: 36301498 - Sex Comb on Midleg Like-2 Accelerates Hepatocellular Carcinoma Cell Proliferation and Metastasis by Activating Wnt/β-Catenin/EMT Signaling.
Du L, Wang L, Yang H, Duan J, Lai J, Wu W, Fan S, Zhi X. Du L, et al. Yonsei Med J. 2021 Dec;62(12):1073-1082. doi: 10.3349/ymj.2021.62.12.1073. Yonsei Med J. 2021. PMID: 34816637 Free PMC article. - Overexpression of malignant brain tumor domain containing protein 1 predicts a poor prognosis of prostate cancer.
Wu W, Bai S, Zhu D, Li K, Dong W, He W, Peng S, Lai Y, Wang Q, Guo Z, Liu L, Huang H. Wu W, et al. Oncol Lett. 2019 May;17(5):4640-4646. doi: 10.3892/ol.2019.10109. Epub 2019 Mar 5. Oncol Lett. 2019. PMID: 30944653 Free PMC article.
References
- Beuchle D, Struhl G, Muller J (2001) Polycomb group proteins and heritable silencing of Drosophila Hox genes. Development 128: 993–1004 - PubMed
- Bornemann D, Miller E, Simon J (1996) The Drosophila Polycomb group gene Sex comb on midleg (Scm) encodes a zinc finger protein with similarity to polyhomeotic protein. Development 122: 1621–1630 - PubMed
- Breen TR, Duncan IM (1986) Maternal expression of genes that regulate the bithorax complex of Drosophila melanogaster. Dev Biol 118: 442–456 - PubMed
- CCP4 (1994) Collaborative Computational Project Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D 50: 760–776 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases