Efficacy of intranasal administration of a truncated NS1 modified live influenza virus vaccine in swine - PubMed (original) (raw)
Randomized Controlled Trial
Efficacy of intranasal administration of a truncated NS1 modified live influenza virus vaccine in swine
Amy L Vincent et al. Vaccine. 2007.
Abstract
In the U.S., despite available swine influenza virus (SIV) vaccines, multiple influenza subtypes as well as antigenic and genetic variants within subtypes continue to circulate in the swine population. One of the challenges to control and eliminate SIV is that the currently used inactivated influenza virus vaccines do not provide adequate cross-protection against multiple antigenic variants of SIV in the field. We previously generated a recombinant H3N2 swine influenza virus (SIV) based on the influenza A/SW/TX/4199-2/98 virus (TX98) containing an NS1 gene expressing a truncated NS1 protein of 126 amino acids, TX98-NS1Delta126 virus. This recombinant strain was demonstrated to be highly attenuated in swine and showed potential for use as a modified live-virus vaccine (MLV) after intratracheal application in pigs. However, this route of inoculation is not practical for vaccination in the field. In the present study, we first compared intramuscular and intranasal routes of application of the MLV, and found that the intranasal route was superior in priming the local (mucosal) immune response. Pigs were then vaccinated via the intranasal route and challenged with wild type homologous TX98 H3N2 virus, with a genetic and antigenic variant H3N2 SIV (influenza A/SW/CO/23619/99 virus, CO99) and a heterosubtypic H1N1 SIV (influenza A/SW/IA/00239/2004 virus, IA04). The intranasally vaccinated pigs were completely protected against homologous challenge. In addition, MLV vaccination provided nearly complete protection against the antigenic H3N2 variant CO99 virus. When challenged with the H1N1 IA04 virus, MLV vaccinated animals displayed reduced fever and virus titers despite minimal reduction in lung lesions. In vaccinated pigs, there was no serologic cross-reactivity by HI assays with the heterologous or heterosubtypic viruses. However, there appeared to be substantial cross-reactivity in antibodies at the mucosal level with the CO99 virus in MLV vaccinated pigs.
Figures
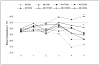
Fig. 1
Mean rectal temperatures from 0 to 5 days post-infection (dpi) in pigs vaccinated 2 times via the intranasal route or non-vaccinated and challenged with homologous and heterologous viruses. Days post-infection are plotted along the X-axis. The cut-off for a febrile response was calculated as two standard deviations above the mean rectal temperature of 0 dpi and sham inoculated pigs from 0 to 5 dpi and is represented by the dashed horizontal line. *Statistical significance at p<0.05 was detected between the non-vaccinated IA04 challenged pigs compared to MLV vaccinated IA04 challenged pigs at 5 dpi.
Similar articles
- Vaccination of pigs against swine influenza viruses by using an NS1-truncated modified live-virus vaccine.
Richt JA, Lekcharoensuk P, Lager KM, Vincent AL, Loiacono CM, Janke BH, Wu WH, Yoon KJ, Webby RJ, Solórzano A, García-Sastre A. Richt JA, et al. J Virol. 2006 Nov;80(22):11009-18. doi: 10.1128/JVI.00787-06. Epub 2006 Aug 30. J Virol. 2006. PMID: 16943300 Free PMC article. - Vaccination with NS1-truncated H3N2 swine influenza virus primes T cells and confers cross-protection against an H1N1 heterosubtypic challenge in pigs.
Kappes MA, Sandbulte MR, Platt R, Wang C, Lager KM, Henningson JN, Lorusso A, Vincent AL, Loving CL, Roth JA, Kehrli ME Jr. Kappes MA, et al. Vaccine. 2012 Jan 5;30(2):280-8. doi: 10.1016/j.vaccine.2011.10.098. Epub 2011 Nov 7. Vaccine. 2012. PMID: 22067263 - Immunogenicity and protective efficacy of an elastase-dependent live attenuated swine influenza virus vaccine administered intranasally in pigs.
Masic A, Lu X, Li J, Mutwiri GK, Babiuk LA, Brown EG, Zhou Y. Masic A, et al. Vaccine. 2010 Oct 8;28(43):7098-108. doi: 10.1016/j.vaccine.2010.08.003. Epub 2010 Aug 12. Vaccine. 2010. PMID: 20708697 - Swine influenza virus vaccines: to change or not to change-that's the question.
Van Reeth K, Ma W. Van Reeth K, et al. Curr Top Microbiol Immunol. 2013;370:173-200. doi: 10.1007/82_2012_266. Curr Top Microbiol Immunol. 2013. PMID: 22976350 Review. - Vaccine development for protecting swine against influenza virus.
Chen Q, Madson D, Miller CL, Harris DL. Chen Q, et al. Anim Health Res Rev. 2012 Dec;13(2):181-95. doi: 10.1017/S1466252312000175. Anim Health Res Rev. 2012. PMID: 23253165 Review.
Cited by
- The impact of maternally derived immunity on influenza A virus transmission in neonatal pig populations.
Allerson M, Deen J, Detmer SE, Gramer MR, Joo HS, Romagosa A, Torremorell M. Allerson M, et al. Vaccine. 2013 Jan 7;31(3):500-5. doi: 10.1016/j.vaccine.2012.11.023. Epub 2012 Nov 20. Vaccine. 2013. PMID: 23174202 Free PMC article. - Alpha-C-galactosylceramide as an adjuvant for a live attenuated influenza virus vaccine.
Kopecky-Bromberg SA, Fraser KA, Pica N, Carnero E, Moran TM, Franck RW, Tsuji M, Palese P. Kopecky-Bromberg SA, et al. Vaccine. 2009 Jun 8;27(28):3766-74. doi: 10.1016/j.vaccine.2009.03.090. Epub 2009 Apr 23. Vaccine. 2009. PMID: 19464560 Free PMC article. - Blocking interhost transmission of influenza virus by vaccination in the guinea pig model.
Lowen AC, Steel J, Mubareka S, Carnero E, García-Sastre A, Palese P. Lowen AC, et al. J Virol. 2009 Apr;83(7):2803-18. doi: 10.1128/JVI.02424-08. Epub 2009 Jan 19. J Virol. 2009. PMID: 19153237 Free PMC article. - Poly I:C adjuvanted inactivated swine influenza vaccine induces heterologous protective immunity in pigs.
Thomas M, Wang Z, Sreenivasan CC, Hause BM, Gourapura J Renukaradhya, Li F, Francis DH, Kaushik RS, Khatri M. Thomas M, et al. Vaccine. 2015 Jan 15;33(4):542-8. doi: 10.1016/j.vaccine.2014.11.034. Epub 2014 Nov 28. Vaccine. 2015. PMID: 25437101 Free PMC article. - Interplay between Janus Kinase/Signal Transducer and Activator of Transcription Signaling Activated by Type I Interferons and Viral Antagonism.
Nan Y, Wu C, Zhang YJ. Nan Y, et al. Front Immunol. 2017 Dec 11;8:1758. doi: 10.3389/fimmu.2017.01758. eCollection 2017. Front Immunol. 2017. PMID: 29312301 Free PMC article. Review.
References
- Belshe RB. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 2004 Jul;103(1–2):177–85. - PubMed
- Townsend HG, Penner SJ, Watts TC, Cook A, Bogdan J, Haines DM, et al. Efficacy of a cold-adapted, intranasal, equine influenza vaccine: challenge trials. Equine veterinary journal. 2001 Nov;33(7):637–43. - PubMed
- Belshe RB, Nichol KL, Black SB, Shinefield H, Cordova J, Walker R, et al. Safety, efficacy, and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5–49 years. Clin Infect Dis. 2004 Oct 1;39(7):920–7. - PubMed
- Buonagurio DA, O’Neill RE, Shutyak L, D’Arco GA, Bechert TM, Kazachkov Y, et al. Genetic and phenotypic stability of cold-adapted influenza viruses in a trivalent vaccine administered to children in a day care setting. Virology. 2006 Apr 10;347(2):296–306. - PubMed
- Chambers TM, Holland RE, Tudor LR, Townsend HG, Cook A, Bogdan J, et al. A new modified live equine influenza virus vaccine: phenotypic stability, restricted spread and efficacy against heterologous virus challenge. Equine veterinary journal. 2001 Nov;33(7):630–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI070469/AI/NIAID NIH HHS/United States
- U01 AI070469-02/AI/NIAID NIH HHS/United States
- U19 AI62623/AI/NIAID NIH HHS/United States
- U19 AI062623-040003/AI/NIAID NIH HHS/United States
- U19 AI062623-030003/AI/NIAID NIH HHS/United States
- U19 AI062623/AI/NIAID NIH HHS/United States
- U01 AI070469-01/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical