Sex-specific DoublesexM expression in subsets of Drosophila somatic gonad cells - PubMed (original) (raw)
Sex-specific DoublesexM expression in subsets of Drosophila somatic gonad cells
Leonie U Hempel et al. BMC Dev Biol. 2007.
Abstract
Background: In Drosophila melanogaster, a pre-mRNA splicing hierarchy controls sexual identity and ultimately leads to sex-specific Doublesex (DSX) transcription factor isoforms. The male-specific DSXM represses genes involved in female development and activates genes involved in male development. Spatial and temporal control of dsx during embryogenesis is not well documented.
Results: Here we show that DSX(M) is specifically expressed in subsets of male somatic gonad cells during embryogenesis. Following testis formation, germ cells remain in contact with DSX(M)-expressing cells, including hub cells and premeiotic somatic cyst cells that surround germ cells during spermatogenesis in larval and adult testes.
Conclusion: We show that dsx is transcriptionally regulated in addition to being regulated at the pre-mRNA splicing level by the sex determination hierarchy. The dsx locus is spatially controlled by somatic gonad identity. The continuous expression of DSX(M) in cells contacting the germline suggests an ongoing short-range influence of the somatic sex determination pathway on germ cell development.
Figures
Figure 1
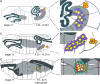
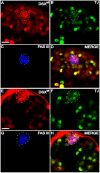
dsx m and DSXM in embryos. (A) RT-PCR of dsx m from sex sorted embryos of ages 3–10 hours; 10–16 hours and 16–22 hours. (B) β3-tubulin amplification control. (C) Cartoon of dsx transcripts. The positions of the primers used for the amplification of the male-specific dsx m products are marked (arrows). (D, F, H) Anti-DSXM and (E, G, I) anti-VASA immunofluorescence in (D, E) female, (F, G) male, and (H, I) _dsx_- embryos (_Df(3R)dsx_15/_In(3R)dsx_23). Images in each row are from the same confocal section of an embryo. Because we used GFP to distinguish homozygous _dsx_- from balancer and heterozygous control embryos, embryos in H, I were not sex sorted. However, we never observed DSXM staining in _dsx_- embryos, 50% of which were male. Secondary antibody for anti-DSXM was biotin-coupled goat anti-rat with tyramide signal amplification, and secondary for anti-VASA was Cy5 goat anti-rabbit. Scale bar = 10 μm.
Figure 2
Gonad development. Foregut and hindgut (dark-gray); anterior and posterior midgut (light-gray); somatic gonadal precursors (purple); germ cells (yellow); male-specific somatic gonadal precursors (orange); somatic gonadal precursors of the hub (red); and a previously undescribed group of cells (green) are indicated. (A) Stage 12 embryo. (B) Higher magnification view of the outlined area in A. (C) Stage 13 embryo. (D) Higher magnification view of the outlined area in C. (E) Stage 17 male embryo. (F) Higher magnification view of the outlined area in E. Cartoons of embryonic gonad development were adapted from Hartenstein [52]. During gonad formation (A, B) the germ cells and the associated somatic gonad precursors co-migrate towards abdominal segment 5, where they begin to coalesce to form the gonads [53, 54]. During and after gonad coalescence (C, D), the germ cells are intermingled with the somatic gonad cells [41]. Prior to gonad coalescence male-specific somatic gonadal precursor cells, specified in parasegment 13 in both males and females, are located posterior and ventral to non-sex-specific somatic gonad precursor cells. During stage 13 these cells move toward the gonad in both sexes, but only in males do these cells join the posterior of the coalescing gonad. In females these cell die, making the surviving ones "male-specific" [21]. The anterior somatic gonad also becomes sexually dimorphic early during gonad development (E, F). The hub, a cluster of somatic cells required for germline stem cell maintenance in the adult testis, forms anteriorly in the male embryonic gonad [29]. Later in stage 17, we saw another group of cells envelop the embryonic testis (E, F). The identity of these cells is uncertain, but they may be the precursors of the testis sheath [17].
Figure 3
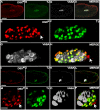
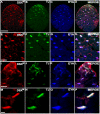
DSXM and TJ expression in the male somatic gonad. (A-H) Stage 13 male embryo immunofluorescence using: (A) anti-DSXM, (B) anti-TJ, and (C) anti-VASA. (D) Merged images A-C. (E-H) Magnified view of the gonad in A-D. Somatic nuclei expressing DSXM but not TJ are indicated (arrows). (I-P) Stage 15 male embryo immunofluorescence using: (I) anti-DSXM, (J) anti-TJ, and (K) anti-VASA. (L) Merged images I-K. (M-P) Magnified view of the gonad in I-L. The scale bars = 50 μm in A-D; I-L and 10 μm in E-H; M-P. Anterior is to the left. Secondary antibodies were: (A, I) biotin-coupled goat anti-rat with TSA, (B, J) Alexa 488 goat anti-guinea pig, and (C, K) Cy5 goat anti-rabbit.
Figure 4
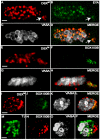
DSXM but not TJ is expressed in male-specific somatic gonadal precursors. (A-D) Stage 13 testis immunofluorescence using (A) anti-DSXM, (B) anti-EYA, and (C) anti-VASA. (D) Merged images A-C. A DSXM and EYA positive cluster of cell nuclei is located posterior and ventral to the other cells of the somatic gonad (arrows). (E-H) Stage 13 male testis immunofluorescence using (E) anti-DSXM, (F) anti-SOX100B and (G) anti-VASA. (H) Merged images E-G. (I-L) Stage 15 testis immunofluorescence using (I) anti-DSXM, (J) anti-SOX100B, and (K) anti-VASA antibody. (L) Merged images I-K. (M-P) Stage 15 testis immunofluorescence using (M) anti-TJ, (N) anti-SOX100B and (O) anti-VASA. (P) Merged images M-O. The scale bars = 10 μm. Anterior is to the left. Secondary antibodies were: (A, E, I) biotin-coupled goat anti-rat and TSA, (B) Alexa 488 goat anti-mouse, (C) Cy5 goat anti-rabbit), (F, J) Alexa 488 goat anti-rabbit, (G, K, O) Alexa 647 goat anti-chicken, (M) Alexa 488 goat anti-guinea pig, (N) biotin-coupled goat anti-rabbit and TSA.
Figure 5
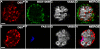
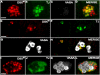
DSXM is not expressed in all somatic testis cells. (A-D) stage 17 testis immunofluorescence using (A) anti-DSXM, (B) anti-SOX100B, and (C) anti-VASA. (D) Merged images A-C. (E-H) stage 17 testis immunofluorescence (imaged in a focal plane with the hub) using (E) anti-DSXM, (F) anti-FAS III, and (G) anti-VASA. (H) Merged images E-G. The scale bars = 10 μm. Secondary antibodies were: (A, E) biotin-coupled goat anti-rat and TSA, (B) Alexa 488 goat anti-rabbit, (C) Alexa 647 goat anti-chicken, (F) Alexa 647 goat anti-mouse, and (G) Cy5 goat anti-rabbit.
Figure 6
DSXM in hub cells. (A-D) Larval (3rd instar) testis immunofluorescence using (A) anti-DSXM, (B) anti-TJ, and (C) anti-FAS III. (D) Merged images A-C. (E-F) Adult testis immunofluorescence using (E) anti-DSXM, (F) anti-TJ, and (G) anti-FAS III. (H) Merged images E-G. The hub is outlined (white dashes). The scale bars = 10 μm. Anterior is up and out of the plane toward the viewer (the outlined hub is most anterior). Secondary antibodies were: (A, E) biotin-coupled goat anti-rat and TSA, (B, F) Alexa 488 goat anti-guinea pig, and (C, G) Alexa 647 goat anti-mouse.
Figure 7
DSXM expression during spermatogenesis. (A-D) Larval testis immunofluorescence using (A) anti-DSXM, (B) anti-TJ, and (C) anti-EYA. (D) Merged images A-C. (E-H) Higher magnification view of A-D. (I-L) Adult apical testis immunofluorescence using (I) anti-DSXM, (J) anti-TJ and (K) anti-EYA. (L) Merged images I-K. (M-P) Higher magnification view of I-L. The scale bar = 50 μm in A-D, 20 μm in E-L, and 5 μm in M-P. Anterior is up. Secondary antibodies were: (A, I) biotin-coupled goat anti-rat and TSA, (B, J) Alexa 488 goat anti-guinea pig, (C, K) Alexa 647 goat anti-mouse.
Figure 8
DSXM expression does not require germ cells nor EYA or TJ. (A-D) Agametic testis of stage 15 males from gs(1)N26 mothers. Immunofluorescence using (A) anti-DSXM, (B) anti-TJ, and (C) anti-VASA. (D) Merged images A-C. (E-H) Isolated somatic gonadal precursors and germ cells formed in homozygous eya mutants. Immunofluorescence using (E) anti-DSXM, (F) anti-TJ, and (G) anti-VASA. (H) Merged images E-G. (I-L) Expression in tj eo mutant testis revealed with immunofluorescence using (I) anti-DSXM, (J) anti-TJ, and (K) anti-VASA. (L) Merged images I-K. Expression of the non-functional truncated TJ protein in tj eo mutant embryos is diffuse within the somatic gonadal precursors (J). The scale bars = 10 μm. Anterior is to the left. Secondary antibodies were: (A, E, I) biotin-coupled goat anti-rat and TSA, (B) Alexa 647 goat anti-guinea pig, (C) Alexa 488 goat anti-rabbit, (F, J) Alexa 488 goat anti-guinea pig, and (G, K) Cy5 goat anti-rabbit.
Figure 9
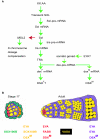
DSXM regulation. DSXM is regulated by the intersection of the sex-determination alternative pre-mRNA splicing hierarchy and spatial/temporal regulation (A). Positive (green arrows) and negative interactions (red) are indicated. See text for details. Cellular markers are differentially expressed in somatic cell types of the male gonad during embryonic and adult stages (B). Cell cartoons and expression indicators are color-coded. Germ cells (yellow), somatic gonad precursors and cyst-cells (purple), male-specific somatic gonadal precursors (orange), hub cells (red), and a novel layer of embryonic testis cells (green) are shown.
Similar articles
- Chinmo prevents transformer alternative splicing to maintain male sex identity.
Grmai L, Hudry B, Miguel-Aliaga I, Bach EA. Grmai L, et al. PLoS Genet. 2018 Feb 1;14(2):e1007203. doi: 10.1371/journal.pgen.1007203. eCollection 2018 Feb. PLoS Genet. 2018. PMID: 29389999 Free PMC article. - Sex determination in the Drosophila germline is dictated by the sexual identity of the surrounding soma.
Waterbury JA, Horabin JI, Bopp D, Schedl P. Waterbury JA, et al. Genetics. 2000 Aug;155(4):1741-56. doi: 10.1093/genetics/155.4.1741. Genetics. 2000. PMID: 10924471 Free PMC article. - Genomic organization and splicing evolution of the doublesex gene, a Drosophila regulator of sexual differentiation, in the dengue and yellow fever mosquito Aedes aegypti.
Salvemini M, Mauro U, Lombardo F, Milano A, Zazzaro V, Arcà B, Polito LC, Saccone G. Salvemini M, et al. BMC Evol Biol. 2011 Feb 10;11:41. doi: 10.1186/1471-2148-11-41. BMC Evol Biol. 2011. PMID: 21310052 Free PMC article. - Double nexus--Doublesex is the connecting element in sex determination.
Verhulst EC, van de Zande L. Verhulst EC, et al. Brief Funct Genomics. 2015 Nov;14(6):396-406. doi: 10.1093/bfgp/elv005. Epub 2015 Mar 22. Brief Funct Genomics. 2015. PMID: 25797692 Free PMC article. Review. - Interplay between sex determination cascade and major signaling pathways during Drosophila eye development: Perspectives for future research.
Surkova S, Görne J, Nuzhdin S, Samsonova M. Surkova S, et al. Dev Biol. 2021 Aug;476:41-52. doi: 10.1016/j.ydbio.2021.03.005. Epub 2021 Mar 18. Dev Biol. 2021. PMID: 33745943 Free PMC article. Review.
Cited by
- Sox100B, a Drosophila group E Sox-domain gene, is required for somatic testis differentiation.
Nanda S, DeFalco TJ, Loh SH, Phochanukul N, Camara N, Van Doren M, Russell S. Nanda S, et al. Sex Dev. 2009;3(1):26-37. doi: 10.1159/000200079. Epub 2009 Apr 1. Sex Dev. 2009. PMID: 19339815 Free PMC article. - Neuropeptide F regulates courtship in Drosophila through a male-specific neuronal circuit.
Liu W, Ganguly A, Huang J, Wang Y, Ni JD, Gurav AS, Aguilar MA, Montell C. Liu W, et al. Elife. 2019 Aug 12;8:e49574. doi: 10.7554/eLife.49574. Elife. 2019. PMID: 31403399 Free PMC article. - Sex and the single embryo: early deveiopment in the Mediterranean fruit fly, Ceratitis capitata.
Gabrieli P, Falaguerra A, Siciliano P, Gomulski LM, Scolari F, Zacharopoulou A, Franz G, Malacrida AR, Gasperi G. Gabrieli P, et al. BMC Dev Biol. 2010 Jan 26;10:12. doi: 10.1186/1471-213X-10-12. BMC Dev Biol. 2010. PMID: 20102629 Free PMC article. - Nonautonomous sex determination controls sexually dimorphic development of the Drosophila gonad.
DeFalco T, Camara N, Le Bras S, Van Doren M. DeFalco T, et al. Dev Cell. 2008 Feb;14(2):275-86. doi: 10.1016/j.devcel.2007.12.005. Dev Cell. 2008. PMID: 18267095 Free PMC article. - Dynamic sex chromosome expression in Drosophila male germ cells.
Mahadevaraju S, Fear JM, Akeju M, Galletta BJ, Pinheiro MMLS, Avelino CC, Cabral-de-Mello DC, Conlon K, Dell'Orso S, Demere Z, Mansuria K, Mendonça CA, Palacios-Gimenez OM, Ross E, Savery M, Yu K, Smith HE, Sartorelli V, Yang H, Rusan NM, Vibranovski MD, Matunis E, Oliver B. Mahadevaraju S, et al. Nat Commun. 2021 Feb 9;12(1):892. doi: 10.1038/s41467-021-20897-y. Nat Commun. 2021. PMID: 33563972 Free PMC article.
References
- Zarkower D. Invertebrates may not be so different after all. Novartis Found Symp. 2002;244:115–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous