Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation - PubMed (original) (raw)
Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation
N Muge Kuyumcu-Martinez et al. Mol Cell. 2007.
Abstract
The genetic basis of myotonic dystrophy type 1 (DM1) is a CTG expansion in the 3' untranslated region (UTR) of DMPK. The pathogenic mechanism involves an RNA gain of function in which the repeat-containing transcripts accumulate in nuclei and alter the functions of RNA-binding proteins such as CUG-binding protein 1 (CUGBP1). CUGBP1 levels are increased in DM1 myoblasts, heart, and skeletal muscle tissues and in some DM1 mouse models. However, the molecular mechanisms for increased CUGBP1 in DM1 are unclear. Here, we demonstrate that expression of DMPK-CUG-repeat RNA results in hyperphosphorylation and stabilization of CUGBP1. CUGBP1 is hyperphosphorylated in DM1 tissues, cells, and a DM1 mouse model. Activation of PKC is required for CUGBP1 hyperphosphorylation in DM1 cells, and PKCalpha and betaII directly phosphorylate CUGBP1 in vitro. These results indicate that inappropriate activation of the PKC pathway contributes to the pathogenic effects of a noncoding RNA.
Figures
Figure 1. DMPK-CUG960 mRNA induces hyper-phosphorylation of CUGBP1
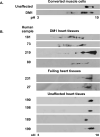
(A) Western blot analysis of hnRNPC (nuclear marker) or GAPDH (cytoplasmic marker) demonstrates a clean separation of nuclear and cytoplasmic extracts used for panel B. (B) Western Blot analysis of nuclear (top panel) or cytoplasmic (bottom panel) CUGBP1 in COS M6 expressing DMPK mRNA containing no repeats (DMPK-CUG0), 960 CAG repeats (DMPK-CAG960) or 960 CUG repeats (DMPK-CUG960). Proteins were separated by 2D gel electrophoresis and CUGBP1 was detected by western blot using monoclonal antibody 3B1. Results shown are representative of least three independent experiments. pH 3 to10 designates the pH range of the strips used in the experiment. (C) CIAP treatment demonstrates that the acidic shift of CUGBP1 is due to hyper-phosphorylation. Untreated, mock treated or CIAP-treated nuclear lysates were analyzed by 2D/western blotting. (D) Flag-CUGBP1 was co-expressed with DMPK-CUG960, DMPK-CUG0 mRNAs or no mRNA. Forty-eight hours post-transfection, nuclear proteins were analyzed on 2D gels and probed with HRP conjugated anti-Flag monoclonal antibodies. (E) Increased steady state levels of hyper-phosphorylated CUGBP1 in nuclear fractions of COS M6 cells expressing 960 CUG repeats. Nuclear or cytoplasmic proteins were separated by 10% SDS-PAGE and membranes were probed for CUGBP1 and GAPDH or hnRNPC as loading controls.
Figure 2. CUGBP1 is hyper-phosphorylated in DM1 heart tissues and converted muscle cells
(A) CUGBP1 hyper-phosphorylation in DM1 muscle cells. DM1 (#3989, 2000 CTG) or normal (#7492) skin fibroblasts were transduced by a retrovirus expressing MyoD and induced to undergo muscle differentiation. Whole cell extracts were analyzed by 2D/western blotting. (B) CUGBP1 hyper-phosphorylation in DM1 human heart tissues. Total cell lysates from DM1 (top panel), cardiomyopathy (middle panel) or normal (bottom panel) heart tissues were analyzed by 2D/western blotting.
Figure 3. CUGBP1 is hyper-phosphorylated in heart tissue from an inducible DM1 mouse model
(A) Heart tissue from bitransgenic animals in which DMPK-CUG960 mRNA expression was induced (EpA960/MCM +Tamoxifen) or not induced (EpA960/MCM + mock) were analyzed by 2D/western blotting. MCM transgenic animals injected with tamoxifen (MCM +Tamoxifen) do not exhibit CUGBP1 hyper-phosphorylation. The results were reproduced in at least three different bitransgenic and transgenic animals. (B) Timing of CUGBP1 hyper-phosphorylation directly correlates with elevated CUGBP1 protein levels. A longer exposure was used for the 6h sample to better detect acidic shifts of CUGBP1.
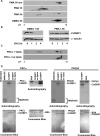
Figure 4. PKC mediated hyper-phosphorylation of CUGBP1 increases protein stability
(A) COS M6 cells were either treated with 30ng/ml PMA or vehicle alone (DMSO) for the indicated times. Whole cell lysates were analyzed by 2D/western blotting. (B) PMA induced hyper-phosphorylation of CUGBP1 correlates with increased half-life. COS M6 cells were treated with PMA (30ng/ml) or DMSO for 1.5 h followed by cycloheximide (10ng/ml) treatment. Cells were harvested at the indicated time points and CUGBP1 levels were determined by western blot. α-tubulin was used as a loading control. To calculate the half-life of CUGBP1, CUGBP1 and α-tubulin signal was quantified from two independent experiments using Kodak Gel Logic 2200 and Molecular Imaging Software. (C) Incubation of His-CUGBP1 with recombinant human PKCα in an in vitro kinase reaction results in an acidic shift detected by 2D/western blotting. PKCα is inactive in the absence of lipids. (D) Both human PKCα and βII directly phosphorylate His-CUGBP1 in a phospholipid dependent manner in vitro. Recombinant PKC isozymes α and βII were incubated with PKC substrate MARCKS peptide, bovine serum albumin (BSA) or His-CUGBP1 in a kinase reaction containing 32P-γ-ATP. Phosphorylated proteins were analyzed on 14% SDS-PAGE gels followed by autoradiography and Coomassie blue staining.
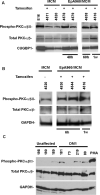
Figure 5. Induction of CUGBP1 hyper-phosphorylation by CUG repeat RNA requires PKC activity
(A) Western blot analysis of total and phospho-PKCα/βII in COS M6 cells expressing DMPK-CUG0, or DMPK-CUG960. hnRNPC was used as an additional loading control. (B) PKC inhibitors blocked CUGBP1 hyper-phosphorylation in COS M6 cells expressing DMPK-CUG960 mRNA. COS M6 were transfected with DMPK-CUG0 or DMPK-CUG960. Forty-four hours post-transfection, cells were either treated with Bis-1, Bis-IX or with vehicle alone (DMSO) for 4h. CUGBP1 was analyzed by 2D/western blotting. (C) Inhibition of CUGBP1 hyper-phosphorylation in DM1 skin fibroblast (#3132, 2000 CTG) treated with Bis-1. DM1 skin fibroblast cultures were either untreated or treated with Bis-1 (PKC inhibitor), U0126 (MEK1/2 inhibitor) or DMSO for 48 h. Normal skin fibroblast (#8402) cultures were either untreated or treated with UO126, Bis-1 or Bis-V (a non-functional analogue of Bis-1) for 48 h. CUGBP1 was analyzed by 2D/western blotting. (D) Inhibition of PKC in DM1 skin fibroblast using Bis-1. The same protein samples were analyzed by 10% SDS-PAGE followed by western blot analysis using phospho-PKCα/βII, GAPDH and hnRNPC antibodies. Total PKCα/βII levels were determined after stripping the membrane probed with phospho-PKCα/βII antibody.
Figure 6. PKCα is activated in DM1
(A-B) PKCα/βII activation in a DM1 mouse model inducibly expressing 960 CUG repeats in heart. Western blot analysis of total and phospho-PKCα/βII and CUGBP1 in normal embryonic day 16 (e16) heart, MCM and EpA960/MCM mice heart tissues. Tamoxifen administration is indicated as (+) or (–). Heart tissues were analyzed at indicated times after tamoxifen injection. GAPDH was used as an additional loading control. (C) PKCα/βII activation in DM1 heart tissues that were analyzed for CUGBP1 phosphorylation in Figure 2. Sample 199 (not shown) failed to show a signal for total PKCα/βII and showed a weak signal for GAPDH. Total and phospho-PKCα/βII levels were determined as described above.
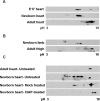
Figure 7. CUGBP1 is hyper-phosphorylated in newborn and embryonic heart and skeletal muscle tissues
CUGBP1 phosphorylation was analyzed on 2D gels (A) during heart development (e17, newborn and 6 months old adult mice), and (B) during skeletal muscle development (newborn and 6 months old adult mice) by western blot using HRP conjugated CUGBP1 monoclonal antibody. Seventy-five micrograms of total protein from e17 and newborn tissues and one hundred fifty micrograms of adult heart tissues were analyzed. Representative results from at least three independent experiments are shown. Note that e17 heart samples were a pool of eight mice hearts. (C) CUGBP1 is hyper-phosphorylated in newborn heart. Untreated, mock treated or CIAP-treated newborn heart tissues were analyzed by 2D/western blotting.
Similar articles
- Correction of RNA-Binding Protein CUGBP1 and GSK3β Signaling as Therapeutic Approach for Congenital and Adult Myotonic Dystrophy Type 1.
Timchenko L. Timchenko L. Int J Mol Sci. 2019 Dec 21;21(1):94. doi: 10.3390/ijms21010094. Int J Mol Sci. 2019. PMID: 31877772 Free PMC article. Review. - Elevation of RNA-binding protein CUGBP1 is an early event in an inducible heart-specific mouse model of myotonic dystrophy.
Wang GS, Kearney DL, De Biasi M, Taffet G, Cooper TA. Wang GS, et al. J Clin Invest. 2007 Oct;117(10):2802-11. doi: 10.1172/JCI32308. J Clin Invest. 2007. PMID: 17823658 Free PMC article. - CUGBP1 overexpression in mouse skeletal muscle reproduces features of myotonic dystrophy type 1.
Ward AJ, Rimer M, Killian JM, Dowling JJ, Cooper TA. Ward AJ, et al. Hum Mol Genet. 2010 Sep 15;19(18):3614-22. doi: 10.1093/hmg/ddq277. Epub 2010 Jul 5. Hum Mol Genet. 2010. PMID: 20603324 Free PMC article. - PKC inhibition ameliorates the cardiac phenotype in a mouse model of myotonic dystrophy type 1.
Wang GS, Kuyumcu-Martinez MN, Sarma S, Mathur N, Wehrens XH, Cooper TA. Wang GS, et al. J Clin Invest. 2009 Dec;119(12):3797-806. doi: 10.1172/JCI37976. Epub 2009 Nov 9. J Clin Invest. 2009. PMID: 19907076 Free PMC article. - Pathogenic mechanisms of myotonic dystrophy.
Lee JE, Cooper TA. Lee JE, et al. Biochem Soc Trans. 2009 Dec;37(Pt 6):1281-6. doi: 10.1042/BST0371281. Biochem Soc Trans. 2009. PMID: 19909263 Free PMC article. Review.
Cited by
- RNase H-mediated degradation of toxic RNA in myotonic dystrophy type 1.
Lee JE, Bennett CF, Cooper TA. Lee JE, et al. Proc Natl Acad Sci U S A. 2012 Mar 13;109(11):4221-6. doi: 10.1073/pnas.1117019109. Epub 2012 Feb 27. Proc Natl Acad Sci U S A. 2012. PMID: 22371589 Free PMC article. - The importance of CELF control: molecular and biological roles of the CUG-BP, Elav-like family of RNA-binding proteins.
Dasgupta T, Ladd AN. Dasgupta T, et al. Wiley Interdiscip Rev RNA. 2012 Jan-Feb;3(1):104-21. doi: 10.1002/wrna.107. Epub 2011 Aug 17. Wiley Interdiscip Rev RNA. 2012. PMID: 22180311 Free PMC article. Review. - Muscleblind participates in RNA toxicity of expanded CAG and CUG repeats in Caenorhabditis elegans.
Wang LC, Chen KY, Pan H, Wu CC, Chen PH, Liao YT, Li C, Huang ML, Hsiao KM. Wang LC, et al. Cell Mol Life Sci. 2011 Apr;68(7):1255-67. doi: 10.1007/s00018-010-0522-4. Epub 2010 Sep 17. Cell Mol Life Sci. 2011. PMID: 20848157 Free PMC article. - Correction of RNA-Binding Protein CUGBP1 and GSK3β Signaling as Therapeutic Approach for Congenital and Adult Myotonic Dystrophy Type 1.
Timchenko L. Timchenko L. Int J Mol Sci. 2019 Dec 21;21(1):94. doi: 10.3390/ijms21010094. Int J Mol Sci. 2019. PMID: 31877772 Free PMC article. Review. - Aberrant alternative splicing and extracellular matrix gene expression in mouse models of myotonic dystrophy.
Du H, Cline MS, Osborne RJ, Tuttle DL, Clark TA, Donohue JP, Hall MP, Shiue L, Swanson MS, Thornton CA, Ares M Jr. Du H, et al. Nat Struct Mol Biol. 2010 Feb;17(2):187-93. doi: 10.1038/nsmb.1720. Epub 2010 Jan 24. Nat Struct Mol Biol. 2010. PMID: 20098426 Free PMC article.
References
- Cardani R, Mancinelli E, Rotondo G, Sansone V, Meola G. Muscleblind-like protein 1 nuclear sequestration is a molecular pathology marker of DM1 and DM2. Eur J Histochem. 2006;50:177–182. - PubMed
- Charlet-B. N, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell. 2002;10:45–53. - PubMed
- Dansithong W, Paul S, Comai L, Reddy S. MBNL1 is the primary determinant of focus formation and aberrant insulin receptor splicing in DM1. J Biol Chem. 2005;280:5773–5780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1F32AR052630-01/AR/NIAMS NIH HHS/United States
- R01 AR045653/AR/NIAMS NIH HHS/United States
- R01 HL045565-17/HL/NHLBI NIH HHS/United States
- R01AR45653/AR/NIAMS NIH HHS/United States
- R01 AR045653-09/AR/NIAMS NIH HHS/United States
- F32 AR052630/AR/NIAMS NIH HHS/United States
- R01HL45565/HL/NHLBI NIH HHS/United States
- R01 HL045565/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials