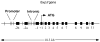Interleukin-2 receptor signaling in regulatory T cell development and homeostasis - PubMed (original) (raw)
Review
Interleukin-2 receptor signaling in regulatory T cell development and homeostasis
Matthew A Burchill et al. Immunol Lett. 2007.
Abstract
Interleukin-2 (IL2) was initially identified from supernatants of activated lymphocytes over 30 years ago. In the ensuing 15 years, the cDNAs for both IL2 and the three chains of the interleukin-2 receptor (IL2R) were cloned. Subsequently, many of the downstream biochemical pathways activated by the IL2 receptor complex were identified and the structure of IL2 bound to this tripartite receptor complex was solved. Thus, we now have a very good understanding of how each chain contributes to high affinity IL2 binding and signal transduction. In contrast, over the past 30 years the role that IL2 plays in regulating lymphocyte function has involved many surprising twists and turns. For example, IL2 has been shown, paradoxically, to regulate both lymphocyte proliferation and lymphocyte death. In this review, we briefly outline the original findings suggesting a role for IL2 as a T cell growth factor, as well as subsequent studies pointing to its function as an initiator of activation-induced cell death, but then focus on the newly appreciated role for IL2 and IL2R signaling in the development and homeostasis of regulatory T cells.
Figures
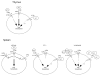
Figure 1. Model of Cytokine-dependent Treg Development and Homeostasis
In the thymus, IL2 plays the predominant role in driving Treg development. In the absence of IL2, IL15 is sufficient for regulatory T cell development. Other γc-dependent cytokines such as IL7 may play a minor role in this process and most likely account for the few Tregs found in _IL2Rβ_−/− mice as compared to _γc_−/− mice in which Tregs are absent. In the spleen of wild type mice, IL2-dependent signals lead to the downregulation of both the IL7Rα and IL15Rα chains. This renders mature Tregs primarily dependent on IL2 for their survival and expansion. In contrast, in _IL2_−/− mice, IL15Rα and IL7Rα remain expressed at high levels and allow for survival of mature Tregs. Likewise, under lymphopenic conditions, such as occurs upon introduction of Tregs into _rag2_−/− mice, Tregs do survive and expand. This most likely occurs due the absence of other T cell subsets thereby leading to increased amounts of available IL15 and IL7 that can stimulate Treg survival despite lower expression of IL15Rα and IL7Rα receptors by those cells. In addition, the absence of IL2 signals may lead to re-expression of the IL15Rα and IL7Rα rendering these cells more sensitive to IL15 and IL7.
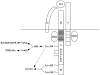
Figure 2. Signaling Components of the IL2 Receptor Complex
The IL2R is composed of three distinct subunits - IL2Rα, IL2Rβ, and IL2Rγ. The IL2Rβ-chain consists of three previously identified signaling domains, the A-, H-, and S-regions. The tyrosine-containing A and H regions are labeled, while the JAK1 binding domain or S-region is shaded in gray. Phosphorylation of Tyr-338 in the A region recruits the adaptor protein SHC; SHC then binds Grb2 and Gab2, leading to activation of the Ras-Raf-MAPK and PI3K/Akt signaling pathways, respectively. Phosphorylation of Tyr-392 and Tyr-510 in the H region recruits STAT5 and leads to its activation; in the absence of these two tyrosine residues Tyr-338 can also induce STAT5 activation.
Figure 3. STAT Binding Motifs in the Murine foxp3 gene
The murine foxp3 gene locus contains many motifs to which activated STAT molecules can bind. Black boxes represent exons while vertical lines represent consensus STAT binding motifs. The three closely linked sites (separated by 7 base pairs) in the promoter and the three sites labeled intronic are conserved across species.
Similar articles
- Interleukin 2-induced tyrosine phosphorylation. Interleukin 2 receptor beta is tyrosine phosphorylated.
Mills GB, May C, McGill M, Fung M, Baker M, Sutherland R, Greene WC. Mills GB, et al. J Biol Chem. 1990 Feb 25;265(6):3561-7. J Biol Chem. 1990. PMID: 2303462 - Role of interleukin 2 receptor beta chain in initiating anti-CD3 and interleukin 2-induced proliferation of human resting T cells.
Moiré N, Calvo CF, Métivier D, Perrot JY, Vaquero C, Hatakeyama M, Senik A. Moiré N, et al. Eur J Immunol. 1990 Sep;20(9):1981-7. doi: 10.1002/eji.1830200916. Eur J Immunol. 1990. PMID: 2145171 - The role of Stat5a and Stat5b in signaling by IL-2 family cytokines.
Lin JX, Leonard WJ. Lin JX, et al. Oncogene. 2000 May 15;19(21):2566-76. doi: 10.1038/sj.onc.1203523. Oncogene. 2000. PMID: 10851055 Review. - Coupling of the IL2 receptor complex with non-receptor protein tyrosine kinases.
Miyazaki T, Taniguchi T. Miyazaki T, et al. Cancer Surv. 1996;27:25-40. Cancer Surv. 1996. PMID: 8909793 Review.
Cited by
- T-cell tolerance in cancer.
Nurieva R, Wang J, Sahoo A. Nurieva R, et al. Immunotherapy. 2013 May;5(5):513-531. doi: 10.2217/imt.13.33. Immunotherapy. 2013. PMID: 23638746 Free PMC article. Review. - Therapeutic cancer vaccines: current status and moving forward.
Schlom J. Schlom J. J Natl Cancer Inst. 2012 Apr 18;104(8):599-613. doi: 10.1093/jnci/djs033. Epub 2012 Mar 6. J Natl Cancer Inst. 2012. PMID: 22395641 Free PMC article. Review. - Identification and comparative expression analysis of interleukin 2/15 receptor β chain in chickens infected with E. tenella.
Jeong J, Kim WH, Yoo J, Lee C, Kim S, Cho JH, Jang HK, Kim DW, Lillehoj HS, Min W. Jeong J, et al. PLoS One. 2012;7(5):e37704. doi: 10.1371/journal.pone.0037704. Epub 2012 May 25. PLoS One. 2012. PMID: 22662196 Free PMC article. - Regulatory T Cells: Molecular Actions on Effector Cells in Immune Regulation.
Arce-Sillas A, Álvarez-Luquín DD, Tamaya-Domínguez B, Gomez-Fuentes S, Trejo-García A, Melo-Salas M, Cárdenas G, Rodríguez-Ramírez J, Adalid-Peralta L. Arce-Sillas A, et al. J Immunol Res. 2016;2016:1720827. doi: 10.1155/2016/1720827. Epub 2016 May 19. J Immunol Res. 2016. PMID: 27298831 Free PMC article. Review. - Abnormal expression pattern of the IL-2 receptor β-chain on CD4+ T cells in ANCA-associated vasculitis.
Wilde B, Hoerning A, Kribben A, Witzke O, Dolff S. Wilde B, et al. Dis Markers. 2014;2014:249846. doi: 10.1155/2014/249846. Epub 2014 Feb 9. Dis Markers. 2014. PMID: 24648606 Free PMC article.
References
- Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. - PubMed
- Taniguchi T, Matsui H, Fujita T, Takaoka C, Kashima N, Yoshimoto R, Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983;302:305–310. - PubMed
- Leonard WJ, Depper JM, Crabtree GR, Rudikoff S, Pumphrey J, Robb RJ, Kronke M, Svetlik PB, Peffer NJ, Waldmann TA, et al. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984;311:626–631. - PubMed
- Nikaido T, Shimizu A, Ishida N, Sabe H, Teshigawara K, Maeda M, Uchiyama T, Yodoi J, Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984;311:631–635. - PubMed
- Cosman D, Cerretti DP, Larsen A, Park L, March C, Dower S, Gillis S, Urdal D. Cloning, sequence and expression of human interleukin-2 receptor. Nature. 1984;312:768–771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007313/AI/NIAID NIH HHS/United States
- AI061165/AI/NIAID NIH HHS/United States
- R01 AI061165-02S1/AI/NIAID NIH HHS/United States
- R01 AI061165-01A1/AI/NIAID NIH HHS/United States
- 2T32-AI07313/AI/NIAID NIH HHS/United States
- R01 AI061165-02/AI/NIAID NIH HHS/United States
- R01 AI061165-03/AI/NIAID NIH HHS/United States
- R56 AI061165/AI/NIAID NIH HHS/United States
- R01 AI061165/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous