Microglial cells in astroglial cultures: a cautionary note - PubMed (original) (raw)
Review
Microglial cells in astroglial cultures: a cautionary note
Josep Saura. J Neuroinflammation. 2007.
Abstract
Primary rodent astroglial-enriched cultures are the most popular model to study astroglial biology in vitro. From the original methods described in the 1970's a great number of minor modifications have been incorporated into these protocols by different laboratories. These protocols result in cultures in which the astrocyte is the predominant cell type, but astrocytes are never 100% of cells in these preparations. The aim of this review is to bring attention to the presence of microglia in astroglial cultures because, in my opinion, the proportion of and the role that microglial cells play in astroglial cultures are often underestimated. The main problem with ignoring microglia in these cultures is that relatively minor amounts of microglia can be responsible for effects observed on cultures in which the astrocyte is the most abundant cell type. If the relative contributions of astrocytes and microglia are not properly assessed an observed effect can be erroneously attributed to the astrocytes. In order to illustrate this point the case of NO production in activated astroglial-enriched cultures is examined. Lipopolysaccharide (LPS) induces nitric oxide (NO) production in astroglial-enriched cultures and this effect is very often attributed to astrocytes. However, a careful review of the published data suggests that LPS-induced NO production in rodent astroglial-enriched cultures is likely to be mainly microglial in origin. This review considers cell culture protocol factors that can affect the proportion of microglial cells in astroglial cultures, strategies to minimize the proportion of microglia in these cultures, and specific markers that allow the determination of such microglial proportions.
Figures
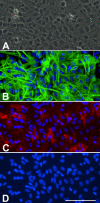
Figure 1
A) Phase contrast image of a confluent murine primary cortical mixed glial culture. A monolayer of type-I astrocytes is observed with some refringent microglia-looking cells. This image could suggest that this is an almost pure astroglial culture. B) GFAP immunostaining of the same field in A seems to confirm this impression. It is difficult to count how many astrocytes are in the field but since the whole field is covered by astrocytes one might conclude that indeed this is an almost pure astroglial culture. C) Immunostaining with the microglial marker CD11b reveals the presence of numerous (20) microglial cells in the field. These are mainly not the round refringent microglial cells typically recovered by shaking and found on top of the astrocytes. Instead these are more ramified cells, in direct contact with the bottom of the well, between the astrocytes or below them. D) Hoechst staining allows the easy quantification of the total number of cells in a culture. In this field there are 109 cells and microglial cells represent 18%. Hoechst 33258 staining also reveals the different nuclear morphology of astrocytes and microglia. Bar, 100 μm
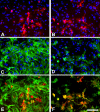
Figure 2
Murine primary cortical mixed glial culture were treated with LPS (1 μg/ml) and IFNγ (0.5 ng/ml) for 24 hours and immunostained for NOS2 (A, B), GFAP (C) or CD11b (D). A and C show the same field and E is their merged image. B and D show the same field and F is their merged image. In control cultures NOS2-immunoreactive cells were not observed (data not shown). There is (LPS + IFNγ)-induced NOS2 expression in numerous cells (A, B). NOS2-positive cells were almost never GFAP immunoreactive (A, C, E) indicating a lack of NOS2 expression in most astrocytes. In contrast, virtually all NOS2-positive cells (>98%) were identified as microglia by their CD11b immunoreactivity. Nuclei are counterstained with Hoechst 33258 in A-D. NOS2-positive cells were identified with a rabbit anti-NOS2 antibody (1:500, BD Biosciences), GFAP-positive cells with a mouse anti-GFAP antibody (1:1000, Sigma) and CD11b-positive cells with 5C6 mouse anti-CD11b antibody (1:400, Serotec). Bar, 100 μm.
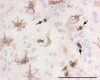
Figure 3
Round and ramified microglia in mixed glial cultures. Bright field image of a murine primary cortical mixed glial culture stained with the microglial marker Tomato lectin (brown) and counterstained with hematoxylin (blue). In this field the proportion of microglial cells is 13%. Three of them, identified with arrows, are round microglial cells with a strong lectin staining. These cells are easily identified by phase contrast by virtue of their refringency. In contrast, there are several microglial cells with ramified morphology and less intense lectin staining. These cells are non-refringent by phase contrast microscopy. Because of their weaker staining with various microglial markers and their non-refringency, the proportion of ramified microglial cells in astroglial-enriched/mixed glial cultures is often underestimated. Microglial cells were identified with biotin-labelled Tomato lectin (1:500, Sigma). Bar, 100 μm.
Similar articles
- Astrocytes enhance lipopolysaccharide-induced nitric oxide production by microglial cells.
Solà C, Casal C, Tusell JM, Serratosa J. Solà C, et al. Eur J Neurosci. 2002 Oct;16(7):1275-83. doi: 10.1046/j.1460-9568.2002.02199.x. Eur J Neurosci. 2002. PMID: 12405988 - Microglial and astroglial reactions to inflammatory lesions of experimental autoimmune encephalomyelitis in the rat central nervous system.
Matsumoto Y, Ohmori K, Fujiwara M. Matsumoto Y, et al. J Neuroimmunol. 1992 Mar;37(1-2):23-33. doi: 10.1016/0165-5728(92)90152-b. J Neuroimmunol. 1992. PMID: 1372328 - Primary culture of cortical neurons, type-1 astrocytes, and microglial cells from cynomolgus monkey (Macaca fascicularis) fetuses.
Negishi T, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. Negishi T, et al. J Neurosci Methods. 2003 Dec 30;131(1-2):133-40. doi: 10.1016/j.jneumeth.2003.08.006. J Neurosci Methods. 2003. PMID: 14659833 - Plasticity of astrocytes in primary cultures: an experimental tool and a reason for methodological caution.
Juurlink BH, Hertz L. Juurlink BH, et al. Dev Neurosci. 1985;7(5-6):263-77. doi: 10.1159/000112295. Dev Neurosci. 1985. PMID: 3915290 Review. - Bioengineered 3D Glial Cell Culture Systems and Applications for Neurodegeneration and Neuroinflammation.
Watson PMD, Kavanagh E, Allenby G, Vassey M. Watson PMD, et al. SLAS Discov. 2017 Jun;22(5):583-601. doi: 10.1177/2472555217691450. Epub 2017 Feb 21. SLAS Discov. 2017. PMID: 28346104 Review.
Cited by
- Isolation methods and characterization of primary rat neurovascular cells.
Floryanzia S, Lee S, Nance E. Floryanzia S, et al. J Biol Eng. 2024 Jul 11;18(1):39. doi: 10.1186/s13036-024-00434-3. J Biol Eng. 2024. PMID: 38992711 Free PMC article. - Human Microglia-Like Cells Differentiated from Monocytes with GM-CSF and IL-34 Show Phagocytosis of α-Synuclein Aggregates and C/EBPβ-Dependent Proinflammatory Activation.
Llaves-López A, Micoli E, Belmonte-Mateos C, Aguilar G, Alba C, Marsal A, Pulido-Salgado M, Rabaneda-Lombarte N, Solà C, Serratosa J, Vidal-Taboada JM, Saura J. Llaves-López A, et al. Mol Neurobiol. 2024 Jun 20. doi: 10.1007/s12035-024-04289-z. Online ahead of print. Mol Neurobiol. 2024. PMID: 38900366 - Microglia in Ischemic Stroke: Pathogenesis Insights and Therapeutic Challenges.
Shui X, Chen J, Fu Z, Zhu H, Tao H, Li Z. Shui X, et al. J Inflamm Res. 2024 May 22;17:3335-3352. doi: 10.2147/JIR.S461795. eCollection 2024. J Inflamm Res. 2024. PMID: 38800598 Free PMC article. Review. - Simvastatin Differentially Modulates Glial Functions in Cultured Cortical and Hypothalamic Astrocytes Derived from Interferon α/β Receptor Knockout mice.
Bobermin LD, Sesterheim P, da Costa DS, Rezena E, Schmitz I, da Silva A, de Moraes ADM, Souza DO, Wyse AT, Leipnitz G, Netto CA, Quincozes-Santos A, Gonçalves CA. Bobermin LD, et al. Neurochem Res. 2024 Mar;49(3):732-743. doi: 10.1007/s11064-023-04073-w. Epub 2023 Dec 8. Neurochem Res. 2024. PMID: 38063948 - Mild Oxidative Stress Induced by Sodium Arsenite Reduces Lipocalin-2 Expression Levels in Cortical Glial Cells.
Cho YJ, Park SH, Ryu KY. Cho YJ, et al. Int J Mol Sci. 2023 Nov 1;24(21):15864. doi: 10.3390/ijms242115864. Int J Mol Sci. 2023. PMID: 37958847 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources