A corkscrew model for dynamin constriction - PubMed (original) (raw)
Comparative Study
A corkscrew model for dynamin constriction
Jason A Mears et al. Structure. 2007 Oct.
Abstract
Numerous vesiculation processes throughout the eukaryotic cell are dependent on the protein dynamin, a large GTPase that constricts lipid bilayers. We have combined X-ray crystallography and cryo-electron microscopy (cryo-EM) data to generate a coherent model of dynamin-mediated membrane constriction. GTPase and pleckstrin homology domains of dynamin were fit to cryo-EM structures of human dynamin helices bound to lipid in nonconstricted and constricted states. Proteolysis and immunogold labeling experiments confirm the topology of dynamin domains predicted from the helical arrays. Based on the fitting, an observed twisting motion of the GTPase, middle, and GTPase effector domains coincides with conformational changes determined by cryo-EM. We propose a corkscrew model for dynamin constriction based on these motions and predict regions of sequence important for dynamin function as potential targets for future mutagenic and structural studies.
Figures
Figure 1. Schematic diagram of dynamin domains and their organization within a helical density
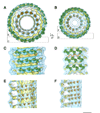
(A) Dynamin contains five functional domains in the primary sequence: GTPase, middle, pleckstrin homology (PH), GTPase effector domain (GED) and proline-rich domain (PRD). (B) Using the same coloring scheme as in (A), the different domains of the primary sequence are highlighted. Additionally, the G-domains of the GTPase are identified with yellow boxes, the variable loops of the PH domain are highlighted with red boxes, the location of the two Shibire mutants (ts1-G273, red; ts2-G146, orange) are identified with black arrows, and unique sequence in the dynamin GTPase are underlined (red and blue, corresponding to ribbon structures seen in Figure 4 A &B). (C) Two sections of the three-dimensional reconstruction of a constricted ΔPRD dynamin helix are presented looking down the helical axis (left) and sectioned (dashed line) and rotated 90° (right) with two density thresholds: blue, low threshold, and yellow, high threshold. The central radial density corresponds to the lipid bilayer (L). Proceeding radially away from the center of the helix, the inner (I), middle (M) and outer (O) radial densities are shown with the cleft and ridge of the helix highlighted. The inner radial density and outer lipid headgroups overlap.
Figure 2. Molecular fittings of the GTPase and PH crystal structures to the non-constricted (A, C & E) and constricted (B, D & F) cryo-EM maps
(A & B) The rat GTPase (green) and human PH (orange) structures fit to the cryo-EM density of the non-constricted (A) and constricted (B) dynamin helix. The density is colored blue and yellow for different thresholds as in Figure 1C. Boxed areas indicate sections for orientations presented in figures 2C–F. (C & D) The organization of the rat GTPase structures is presented normal to the helical axis for a peripheral section of the helical density. The red line highlights the conformational change that occurs in the middle radial density. A dashed box highlights the GTPase dimer density. (E & F) The organization of the human PH structure is presented normal to the helical axis for an interior section (near the lipid bilayer) of the helical density. Dashed boxes and arrow highlight the motions observed between adjacent PH structures. Scale bar, 10 nm.
Figure 3. T-view (cross-section) of the non-constricted and constricted maps with the fit of the GTPase and PH domain crystal structures
(A) A cross-section of the non-constricted helical density is presented with dimers of the GTPase (green and grey ribbon) and PH domain (orange ribbon) crystal structures. The N- and C- termini of the GTPase domain (purple) are highlighted. The variable loops (VL1, VL2, and VL3) of the PH domain are highlighted near the lipid interface. The location of the Shibire ts1 (G273) mutant is seen as a red sphere in each GTPase monomer. Inset: Fitting the Dictyostelium dyn A structure to the cryo-EM density shows a similar fit, but the density cannot accommodate the additional sequence in the dyn A structure (highlighted by red circle). (B) A similar cross-section of the constricted helical density is presented using the same coloring as in (A). Scale bar, 10 nm.
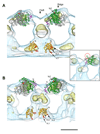
Figure 4. Global reorientation of the GTPase domain upon constriction
(A & B) Four dimers of the GTPase domain fit to the non-constricted (A) and constricted (B) densities are shown. There are two interfaces of interaction highlighted by different ribbon colors. The gold ribbon indicates interface #1 and the red and blue ribbons at interface #2 correspond to the unique dynamin sequence underlined in figure 1B. Inserts highlight the orientation of GTPase structures relative to the helical array (grey densities with red box). (C) Superposition of non-constricted (green) and constricted (blue) monomers indicate relative motions of adjacent monomers at both interfaces. The nucleotide is modeled at the corresponding binding sites (yellow). Both shibire mutants (ts1, G273 is red; ts2, G146 is orange) are shown as spheres in the structures. A dynamin-specific region that potentially interacts after constriction is colored purple (loop).
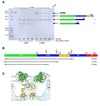
Figure 5. Dynamin proteolysis with trypsin indicates conformation-dependent protections of dynamin
(A) Trypsin digestion of wild-type dynamin in the presence (+ lipid) and absence (− lipid) of PS liposomes. The first lane is dynamin before proteolysis and last lane is a trypsin alone control. Schematic diagram of tryptic fragments are presented (right). (B) Summary of the tryptic fragments of dynamin (ΔPRD, 1–3) determined by mass spectrophotometry. Yellow arrowheads indicate regions exposed to protease in the presence of lipid. The green arrowhead represents cleavage only in the absence of lipid. (C) A cross-section of the ΔPRD dynamin 3D map indicates where the cleavage sites (arrowheads) are likely to occur based on the molecular fittings.
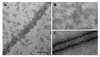
Figure 6. Immunogold labeling of ΔPRD dynamin tubes identifies the location of the GTPase domain
(A & B) ΔPRD dynamin protein labeled with the anti-dynamin, MC65, antibody (Henley et al., 1998) in the presence (A) and absence (B) of liposomes. (C) No labeling is detected with the secondary anti-rabbit 6 nm gold conjugate antibody alone. Scale bar, 100 nm.
Figure 7. A corkscrew model for dynamin constriction
The placement of individual GTPase monomers, shown as green ovals with and without GTP bound (yellow and white small circles, respectively), illustrates the observed motions that occur upon constriction. Specifically, a twisting motion (red dashed arrow) of adjacent dimer structures allows for tighter packing of the GTPase domains affecting interactions with adjacent structures and decreasing the pitch of the helix (106 Å in non-constricted vs. 94 Å in constricted). Furthermore, these motions correspond with the conformational changes observed in the middle radial density where the density is straighter in the non-constricted helix and becomes kinked in the constricted helix (traced with red line). These motions work in unison to twist like corkscrews that constrict and compress the lipid bilayer.
Similar articles
- The stalk region of dynamin drives the constriction of dynamin tubes.
Chen YJ, Zhang P, Egelman EH, Hinshaw JE. Chen YJ, et al. Nat Struct Mol Biol. 2004 Jun;11(6):574-5. doi: 10.1038/nsmb762. Epub 2004 May 9. Nat Struct Mol Biol. 2004. PMID: 15133500 - Cryo-EM structures of membrane-bound dynamin in a post-hydrolysis state primed for membrane fission.
Jimah JR, Kundu N, Stanton AE, Sochacki KA, Canagarajah B, Chan L, Strub MP, Wang H, Taraska JW, Hinshaw JE. Jimah JR, et al. Dev Cell. 2024 Jul 22;59(14):1783-1793.e5. doi: 10.1016/j.devcel.2024.04.008. Epub 2024 Apr 24. Dev Cell. 2024. PMID: 38663399 - The mechanoenzymatic core of dynamin-related protein 1 comprises the minimal machinery required for membrane constriction.
Francy CA, Alvarez FJ, Zhou L, Ramachandran R, Mears JA. Francy CA, et al. J Biol Chem. 2015 May 1;290(18):11692-703. doi: 10.1074/jbc.M114.610881. Epub 2015 Mar 13. J Biol Chem. 2015. PMID: 25770210 Free PMC article. - Dynamin: functional design of a membrane fission catalyst.
Schmid SL, Frolov VA. Schmid SL, et al. Annu Rev Cell Dev Biol. 2011;27:79-105. doi: 10.1146/annurev-cellbio-100109-104016. Epub 2011 May 18. Annu Rev Cell Dev Biol. 2011. PMID: 21599493 Review. - Dynamin, a membrane-remodelling GTPase.
Ferguson SM, De Camilli P. Ferguson SM, et al. Nat Rev Mol Cell Biol. 2012 Jan 11;13(2):75-88. doi: 10.1038/nrm3266. Nat Rev Mol Cell Biol. 2012. PMID: 22233676 Free PMC article. Review.
Cited by
- NMR derived model of GTPase effector domain (GED) self association: relevance to dynamin assembly.
Chakraborty S, Pratihar S, Hosur RV. Chakraborty S, et al. PLoS One. 2012;7(1):e30109. doi: 10.1371/journal.pone.0030109. Epub 2012 Jan 12. PLoS One. 2012. PMID: 22253896 Free PMC article. - Invited review: Mechanisms of GTP hydrolysis and conformational transitions in the dynamin superfamily.
Daumke O, Praefcke GJ. Daumke O, et al. Biopolymers. 2016 Aug;105(8):580-93. doi: 10.1002/bip.22855. Biopolymers. 2016. PMID: 27062152 Free PMC article. Review. - Structural insights into oligomerization and mitochondrial remodelling of dynamin 1-like protein.
Fröhlich C, Grabiger S, Schwefel D, Faelber K, Rosenbaum E, Mears J, Rocks O, Daumke O. Fröhlich C, et al. EMBO J. 2013 May 2;32(9):1280-92. doi: 10.1038/emboj.2013.74. Epub 2013 Apr 12. EMBO J. 2013. PMID: 23584531 Free PMC article. - An intramolecular signaling element that modulates dynamin function in vitro and in vivo.
Chappie JS, Acharya S, Liu YW, Leonard M, Pucadyil TJ, Schmid SL. Chappie JS, et al. Mol Biol Cell. 2009 Aug;20(15):3561-71. doi: 10.1091/mbc.e09-04-0318. Epub 2009 Jun 10. Mol Biol Cell. 2009. PMID: 19515832 Free PMC article. - Stalk domain of the dynamin-like MxA GTPase protein mediates membrane binding and liposome tubulation via the unstructured L4 loop.
von der Malsburg A, Abutbul-Ionita I, Haller O, Kochs G, Danino D. von der Malsburg A, et al. J Biol Chem. 2011 Oct 28;286(43):37858-65. doi: 10.1074/jbc.M111.249037. Epub 2011 Sep 7. J Biol Chem. 2011. PMID: 21900240 Free PMC article.
References
- Binns DD, Barylko B, Grichine N, Atkinson MA, Helms MK, Jameson DM, Eccleston JF, Albanesi JP. Correlation between self-association modes and GTPase activation of dynamin. J Protein Chem. 1999;18:277–290. - PubMed
- Bitoun M, Maugenre S, Jeannet PY, Lacene E, Ferrer X, Laforet P, Martin JJ, Laporte J, Lochmuller H, Beggs AH, et al. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat Genet. 2005;37:1207–1209. - PubMed
- Burger KN, Demel RA, Schmid SL, de Kruijff B. Dynamin is membrane-active: lipid insertion is induced by phosphoinositides and phosphatidic acid. Biochemistry. 2000;39:12485–12493. - PubMed
- Carr JF, Hinshaw JE. Dynamin assembles into spirals under physiological salt conditions upon the addition of GDP and gamma-phosphate analogues. J Biol Chem. 1997;272:28030–28035. - PubMed
- Carson M. Ribbons. Methods Enzymol. 1997;277:493–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources