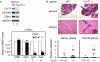AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells - PubMed (original) (raw)
AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells
Irena Oven et al. Mol Cell Biol. 2007 Dec.
Abstract
AIRE is a transcriptional activator that directs the ectopic expression of many tissue-specific genes in medullary thymic epithelial cells, which plays an important role in the negative selection of autoreactive T cells. However, its mechanism of action remains poorly understood. In this study, we found that AIRE regulates the step of elongation rather than initiation of RNA polymerase II. For these effects, AIRE bound and recruited P-TEFb to target promoters in medullary thymic epithelial cells. In these cells, AIRE activated the ectopic transcription of insulin and salivary protein 1 genes. Indeed, by chromatin immunoprecipitation, we found that RNA polymerase II was already engaged on these promoters but was unable to elongate in the absence of AIRE. Moreover, the genetic inactivation of cyclin T1 from P-TEFb abolished the transcription of AIRE-responsive genes and led to lymphocytic infiltration of lacrimal and salivary glands in the CycT1-/- mouse. Our findings reveal critical steps by which AIRE regulates the transcription of genes that control central tolerance in the thymus.
Figures
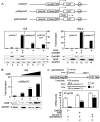
FIG. 1.
AIRE cooperates with initiation factors to activate transcription. (A) (Top) Schematic representations of plasmid targets used in CAT assays. pG5CAT contains the CAT gene and five repeats of the GAL4 DNA-binding site (5×UAS) in front of the E1b TATA box (T). pG6SpCAT contains a complete promoter of three Sp1 sites (3×Sp1) and the E1b TATA box in addition to six UASs and the CAT gene. pG6TARCAT contains a TAR RNA structure in addition to the elements present in pG6SpCAT. pA represents the polyadenylation signal. (Bottom) AIRE requires the presence of initiation factors to activate transcription in 1C6 and HeLa-MAGI cells. Cells expressed the indicated plasmid targets alone or with AIRE (lanes 3, 4, 6, 8, 10, 12, and 14) and GalDBD (lanes 2, 4, 11, and 12). Expression levels of AIRE and GalDBD, as determined by Western blotting, are presented below the CAT data. Levels of endogenous GAPDH were determined by Western blotting to validate input for each sample. Error bars denote standard errors of the means of three independent experiments. (B) AIRE activates transcription in a dose-dependent manner. 1C6 cells expressed pG6SpCAT and increasing amounts of AIRE (0.1, 0.25, and 0.8 μg in lanes 2, 3, and 4, respectively). Expression levels of AIRE and GAPDH, as determined by Western blotting, are presented below the CAT data. Error bars denote standard errors of the means of three independent experiments. (C) AIRE induces the elongation of transcription. (Top) Primer combinations for the amplification of primary transcripts. Primers 1 and 2 amplify TAR and thus all transcripts (ST). Primers 1 and 3 amplify only the LT. nt, nucleotides. (Bottom) Total RNA was extracted from HeLa-MAGI cells coexpressing pG6TARCAT and AIRE or GalDBD and was analyzed by RT-qPCR. The Gal.CycT1 chimera was used as the positive control. Data are representative of three independent experiments.
FIG. 2.
AIRE binds CycT1 in vitro and in cells. (A) AIRE binds the GST.CycT1 chimera in vitro. Binding reactions were performed between GST and the GST.CycT1 chimera, which were expressed in Escherichia coli, and AIRE, which was expressed in HeLa-MAGI cells. (Left) Lanes 1 to 4 contain specific pulldowns, and lane 6 contains 10% of the input AIRE protein. (Right) Input of GST proteins, which were visualized by Coomassie blue staining of SDS-PAGE. Molecular size markers (in kilodaltons) are given in lane M. (B) AIRE interacts with the endogenous CycT1 and Cdk9 proteins in 1C6 cells. The Flag epitope-tagged AIRE protein (lanes 1 to 6) was expressed in 1C6 cells. Total cell lysates were immunoprecipitated (IP) with αFlag M2 agarose beads (lane 2), αCycT1 (lane 5), or mouse IgG (lanes 3 and 6) antibodies and examined for the presence of CycT1, Cdk9, Cdk7, and AIRE by Western blotting (WB) with αCycT1, αCdk9, αCdk7, and αFlag antibodies, respectively. Lanes 1 and 4 represent 10% of the input of indicated proteins.
FIG. 3.
HEXIM1 inhibits the transcriptional activity of AIRE. (A) Increased levels of HEXIM1 block AIRE-mediated transcription from pG6SpCAT. 1C6 cells coexpressed AIRE and increasing amounts of HEXIM1 (0.125, 0.25, and 0.5 μg). CAT assays were performed 24 h after transfection. CAT activity of the plasmid target alone is given as 1 (white bar). Black bars represent activation (_n_-fold) by AIRE (lane 3). In the presence of increasing amounts of HEXIM1, the activity of AIRE decreases (lanes 4 to 6). Below the bar graphs are presented levels of AIRE, HEXIM1, and GAPDH as determined by Western blotting. Error bars denote standard errors of the means of three independent experiments. (B) Depletion of endogenous HEXIM1 protein increases the transcriptional activity of AIRE. 1C6 cells were transfected with mock siRNA (lanes 1 and 2) or siRNA-Hex1 (lanes 3 and 4). The next day, cells were cotransfected with pG6SpCAT and the plasmid encoding MycAIRE (lanes 2 and 4) or the empty plasmid vector as the control (lanes 1 and 3). After an additional 24 h, CAT assays were performed. Amounts of AIRE, endogenous HEXIM1, and GAPDH proteins after siRNA treatment were assessed by immunoblotting and are presented below the bar graph. Error bars denote standard errors of the means of three independent experiments.
FIG. 4.
AIRE activates the transcription of Ins and Spt1 genes in mTECs. (A) AIRE activates transcription from the human Ins promoter. 1C6 cells coexpressed AIRE and the plasmid target containing the human Ins promoter linked to the firefly luciferase gene, and luciferase activity was measured. Renilla luciferase readings were used to normalize the firefly luciferase activity of each sample for all transfections. The expression of AIRE was confirmed by Western blotting and is presented below the bar graph. Error bars denote standard errors of the means of three independent transfections. (B) AIRE activates expression of Ins2 and Spt1 genes in mTECs. RNA was extracted from 1C6 cells transiently expressing AIRE (lanes 1 to 4) or the empty plasmid vector (lanes 5 to 8) and treated additionally with 100 mM TSA for 12 h or from AIRE.1C6 cells that stably expressed AIRE (lane 9). Semiquantitative RT-PCR (fourfold serial dilution) analyses of several tissue-specific genes were performed. PCR was carried out using gene-specific primers as indicated. (C) AIRE mRNA levels in mTECs. RNA was isolated from 1C6 cells (lane 1), primary (1°) mTECs (lane 2), and transiently (lane 3) or stably (lane 4) transfected 1C6 cells, and RT-qPCR was performed with primers specific for AIRE. mRNA levels were normalized to actin.
FIG. 5.
AIRE recruits P-TEFb to Ins2 and Spt1 promoters and stimulates transcriptional elongation by RNAPII. 1C6 (−AIRE) and 1C6.AIRE (+AIRE) cells were analyzed. Formaldehyde-fixed and sonicated chromatin extracts were immunoprecipitated with the indicated antibodies. ChIP/qPCR was performed with the indicated primers to determine the amounts of DNA associated with immunoprecipitated proteins on promoters or coding sequences. The positions of primers are indicated in the diagrams above the graphs. We looked for the presence of AIRE, RNAPII, and subunits of P-TEFb on AIRE-responsive genes (A and B) and AIRE-nonresponsive genes (C and D). ChIP/qPCR with rabbit IgG antibodies was used as the negative control for specificity. All values are expressed relative to the control input DNA (% input) and represent experiments performed in triplicate, with errors indicated.
FIG. 6.
CycT1−/− mice do not express AIRE-responsive genes in the thymus and display lymphocytic infiltration of lacrimal and salivary glands. (A) Absent expression of CycT1 in the thymus (see Fig. S3 in the supplemental material) parallels the lack of Spt1 and Ins2 transcripts in CycT1−/− mice. We assessed levels of CycT1, HEXIM1, Cdk9, and GAPDH in the thymuses from WT or CycT1−/− mice by Western blotting (top). Next, primary mTECs were isolated from the thymuses of WT or CycT1−/− mice, followed by isolation of total RNA and analyses by RT-qPCR with primers specific for AIRE, actin, Spt1, and Ins2 transcripts (bottom, l to 4). WT and CycT1−/− denote parental CycT1+/+ and genetically inactivated CycT1−/− mice, respectively. Amounts of RNA are expressed relative to those in WT mTECs, with standard errors from three independent measurements. (B) Lack of CycT1 in the mouse results in lymphocytic infiltration of lacrimal and salivary glands. Hematoxylin and eosin staining of formalin-fixed sections of lacrimal and salivary glands from 5- to 7-month-old WT and CycT1−/− mice is presented. Arrows point to lymphocytic infiltrates in these organs. Photographs were obtained at ×40 and ×100 (insets) magnifications. Below histological sections are presented relative infiltrations of salivary and lacrimal glands of up to 10 CycT1+/+ and CycT1+/− (WT) and 21 CycT1−/− mice. Scoring was performed blindly, as described previously (18).
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Patient mutation in AIRE disrupts P-TEFb binding and target gene transcription.
Žumer K, Plemenitaš A, Saksela K, Peterlin BM. Žumer K, et al. Nucleic Acids Res. 2011 Oct;39(18):7908-19. doi: 10.1093/nar/gkr527. Epub 2011 Jun 30. Nucleic Acids Res. 2011. PMID: 21724609 Free PMC article. - Expression of autoimmune regulator gene (AIRE) and T regulatory cells in human thymomas.
Scarpino S, Di Napoli A, Stoppacciaro A, Antonelli M, Pilozzi E, Chiarle R, Palestro G, Marino M, Facciolo F, Rendina EA, Webster KE, Kinkel SA, Scott HS, Ruco L. Scarpino S, et al. Clin Exp Immunol. 2007 Sep;149(3):504-12. doi: 10.1111/j.1365-2249.2007.03442.x. Epub 2007 Jun 22. Clin Exp Immunol. 2007. PMID: 17590173 Free PMC article. - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - Adverse effects of immunotherapies for multiple sclerosis: a network meta-analysis.
Tramacere I, Virgili G, Perduca V, Lucenteforte E, Benedetti MD, Capobussi M, Castellini G, Frau S, Gonzalez-Lorenzo M, Featherstone R, Filippini G. Tramacere I, et al. Cochrane Database Syst Rev. 2023 Nov 30;11(11):CD012186. doi: 10.1002/14651858.CD012186.pub2. Cochrane Database Syst Rev. 2023. PMID: 38032059 Free PMC article. Review.
Cited by
- APECED: A Paradigm of Complex Interactions between Genetic Background and Susceptibility Factors.
De Martino L, Capalbo D, Improda N, D'Elia F, Di Mase R, D'Assante R, D'Acunzo I, Pignata C, Salerno M. De Martino L, et al. Front Immunol. 2013 Oct 23;4:331. doi: 10.3389/fimmu.2013.00331. Front Immunol. 2013. PMID: 24167503 Free PMC article. Review. - Tumor emergence is sensed by self-specific CD44hi memory Tregs that create a dominant tolerogenic environment for tumors in mice.
Darrasse-Jèze G, Bergot AS, Durgeau A, Billiard F, Salomon BL, Cohen JL, Bellier B, Podsypanina K, Klatzmann D. Darrasse-Jèze G, et al. J Clin Invest. 2009 Sep;119(9):2648-62. doi: 10.1172/JCI36628. Epub 2009 Aug 3. J Clin Invest. 2009. PMID: 19652360 Free PMC article. - Immune tolerance and the prevention of autoimmune diseases essentially depend on thymic tissue homeostasis.
Shirafkan F, Hensel L, Rattay K. Shirafkan F, et al. Front Immunol. 2024 Mar 20;15:1339714. doi: 10.3389/fimmu.2024.1339714. eCollection 2024. Front Immunol. 2024. PMID: 38571951 Free PMC article. Review. - Central tolerance to self revealed by the autoimmune regulator.
Chan AY, Anderson MS. Chan AY, et al. Ann N Y Acad Sci. 2015 Nov;1356(1):80-9. doi: 10.1111/nyas.12960. Ann N Y Acad Sci. 2015. PMID: 26579596 Free PMC article. Review. - AIRE relies on Z-DNA to flag gene targets for thymic T cell tolerization.
Fang Y, Bansal K, Mostafavi S, Benoist C, Mathis D. Fang Y, et al. Nature. 2024 Apr;628(8007):400-407. doi: 10.1038/s41586-024-07169-7. Epub 2024 Mar 13. Nature. 2024. PMID: 38480882 Free PMC article.
References
- Aasland, R., T. J. Gibson, and A. F. Stewart. 1995. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem. Sci. 20:56-59. - PubMed
- Adams, M., L. Sharmeen, J. Kimpton, J. Romeo, J. Garcia, B. Peterlin, M. Groudine, and M. Emerman. 1994. Cellular latency in human immunodeficiency virus-infected individuals with high CD4 levels can be detected by the presence of promoter-proximal transcripts. Proc. Natl. Acad. Sci. USA 91:3862-3866. - PMC - PubMed
- Anderson, M. S., E. S. Venanzi, Z. Chen, S. P. Berzins, C. Benoist, and D. Mathis. 2005. The cellular mechanism of Aire control of T cell tolerance. Immunity 23:227-239. - PubMed
- Anderson, M. S., E. S. Venanzi, L. Klein, Z. Chen, S. P. Berzins, S. J. Turley, H. von Boehmer, R. Bronson, A. Dierich, C. Benoist, and D. Mathis. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science 298:1395-1401. - PubMed
- Bjorses, P., M. Pelto-Huikko, J. Kaukonen, J. Aaltonen, L. Peltonen, and I. Ulmanen. 1999. Localization of the APECED protein in distinct nuclear structures. Hum. Mol. Genet. 8:259-266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials