Histone deacetylase 6 regulates growth factor-induced actin remodeling and endocytosis - PubMed (original) (raw)
Histone deacetylase 6 regulates growth factor-induced actin remodeling and endocytosis
Ya-sheng Gao et al. Mol Cell Biol. 2007 Dec.
Abstract
Histone deacetylase 6 (HDAC6) is a cytoplasmic deacetylase that uniquely catalyzes alpha-tubulin deacetylation and promotes cell motility. However, the mechanism underlying HDAC6-dependent cell migration and the role for microtubule acetylation in motility are not known. Here we show that HDAC6-induced global microtubule deacetylation was not sufficient to stimulate cell migration. Unexpectedly, in response to growth factor stimulation, HDAC6 underwent rapid translocation to actin-enriched membrane ruffles and subsequently became associated with macropinosomes, the vesicles for fluid-phase endocytosis. Supporting the importance of these associations, membrane ruffle formation, macropinocytosis, and cell migration were all impaired in HDAC6-deficient cells. Conversely, elevated HDAC6 levels promoted membrane ruffle formation with a concomitant increase in macropinocytosis and motility. In search for an HDAC6 target, we found that heat shock protein 90 (Hsp90), another prominent substrate of HDAC6, was also recruited to membrane ruffles and macropinosomes. Significantly, inhibition of Hsp90 activity suppressed membrane ruffling and cell migration, while expression of an acetylation-resistant Hsp90 mutant promoted ruffle formation. Our results uncover a surprising role for HDAC6 in actin remodeling-dependent processes and identify the actin cytoskeleton as an important target of HDAC6-regulated protein deacetylation.
Figures
FIG. 1.
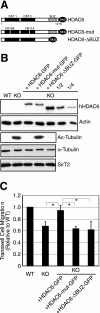
Accumulation of hyperacetylated microtubules in HDAC6 KO tissues and MEFs. (A) A schematic diagram of the targeting strategy with mouse HDAC6 genomic sequences. Exons (vertical bars) 10 to 13 were replaced by a targeting vector containing a neomycin (Neo) and zeocin (Zeo) cassette, resulting in the disruption of the first catalytic domain of HDAC6. The sequences are not drawn to scale. (B and C) Immunoblotting analysis of brain samples (B) or MEFs (C) from wild-type (WT) or HDAC6 KO mice revealed the absence of HDAC6 protein and significantly elevated tubulin acetylation in HDAC6 KO animals. The relative amount of acetylated α-tubulin (Ac-Tubulin) is provided under panel C. p150glued was shown as a loading control. (D) Wild-type (a and b) and HDAC6 KO (c and d) MEFs were immunostained with antibodies for acetylated α-tubulin (b and d) and α-tubulin (a and c). Note that almost the entire microtubule network in HDAC6 KO MEFs was stained positive for acetylated α-tubulin. Bar = 10 μm. (E) Cell motility of wild-type and KO MEFs was analyzed by Boyden chamber cell migration assays. Average values with standard errors of the means from four independent experiments are shown.
FIG. 2.
Both enzymatic activity and the BUZ domain of HDAC6 are required to rescue motility defects of HDAC6 KO cells. (A) Schematic representation of the human HDAC6 constructs used in this study. The constructs are tagged either with GFP or with FLAG. Boxes in black, catalytic domains; boxes in gray, BUZ domain. H216A and H611A are histidine-to-alanine point mutations that inactivate the deacetylase activity of HDAC6. (B) Wild-type, HDAC6 KO, and HDAC6 KO MEFs stably expressing the human HDAC6 constructs were analyzed for the level of HDAC6 in the cells using an anti-human HDAC6 antibody (upper panel). In addition, the HDAC6-ΔBUZ-GFP sample was also serially diluted by two- and fourfold for analysis. Total and acetylated α-tubulin and SirT2 levels were examined using corresponding antibodies. (C) Quantification of cell migration using Boyden chamber assays for cell lines described for panel B. Each bar represents an average value plus standard error of the mean from four independent experiments. *, t < 0.05 in two-tailed and paired t test.
FIG. 3.
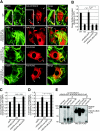
HDAC6 translocates to actin membrane ruffles and is required for efficient ruffle formation. (A) Wild-type (WT; top panel) or HDAC6 KO MEFs that stably express human HDAC6-FLAG, HDAC6-mut-FLAG, or HDAC6-ΔBUZ-FLAG were stimulated with PDGF-BB (50 ng/ml for 8 min) and stained with phalloidin-Alexa Fluor 488 for F-actin (green) or with an antibody for HDAC6 or FLAG. Endogenous HDAC6 (red, anti-mHDAC6) and FLAG-tagged HDAC6 (red, anti-FLAG) are both detected at dorsal (filled arrows) and peripheral (filled arrowheads, second panel from top) membrane ruffles. Open arrows indicate stress fibers (top panel). Insert in top panel: an enlarged image shows the concentration of HDAC6 at F-actin-containing spots (open arrowheads). Bar = 10 μm. (B) Quantification of PDGF-BB-induced dorsal ruffle formation in various cell types. The graph represents cells with dorsal circular ruffles. Approximately 500 cells were examined from each group. Results are averages plus standard errors of the means. n = 5 for WT and KO cells; n = 4 for reconstituted cells. **, t < 0.01 in two-tailed t test. (C) Wild-type MEFs that stably express GFP control, HDAC6-GFP, HDAC6-mut-GFP, or HDAC6-ΔBUZ-GFP were examined for dorsal ruffle formation as for panel B. Each bar represents the average value plus standard error of the mean from four independent experiments. **, t < 0.01 in a two-tailed and paired t test. (D) Cell lines described for panel C were tested in Boyden chamber migration assays, and relative motility values are graphed. Each bar represents the average value plus standard deviation from three independent experiments with duplicate wells. *, t < 0.05 in a two-tailed and paired t test. (E) 293T cells were cotransfected with HDAC6-ΔBUZ-FLAG and HDAC6-GFP and processed for immunoprecipitation using anti-FLAG, anti-GFP, or control mouse antibody. The precipitates were analyzed by immunoblotting using anti-GFP (left panel) or anti-FLAG (right panel) antibody to show mutual association of HDAC6-ΔBUZ and the full-length HDAC6.
FIG. 4.
HDAC6 regulates macropinocytosis. (A) Dorsal ruffle formation and macropinocytic uptake were visualized in HDAC6 KO MEFs that stably express human HDAC6-FLAG. The cells were stimulated with 20 ng/ml PDGF-BB in the presence of 3 mg/ml dextran (70 kDa)-tetramethylrhodamine for 10 min. Triple labeling using anti-hHDAC6 and phalloidin-Alexa Fluor 647 revealed colocalization of HDAC6 (green) and F-actin (blue) at the dextran-positive macropinosomes (red). Arrows point to macropinosomes. Bar = 10 μm. (B) Wild-type MEFs were treated with 100 nM wortmannin or vehicle control for 30 min at 37°C. Cells were stimulated with PDGF-BB in the presence of Dextran-tetramethylrhodamine and wortmannin (or vehicle) for 10 min. The cells were then fixed and stained for F-actin using phalloidin-Alexa Fluor 488. Wortmannin significantly inhibited macropinocytosis of dextran. The arrow indicates a macropinosome. Bar = 10 μm. (C) Fluorocytometric analysis revealed a significant decrease in macropinocytosis of dextran (Dex)-FITC in HDAC6 KO MEFs. Values are averages plus standard errors of the means from four independent experiments. (D) HDAC6 KO MEFs and KO cells stably expressing human HDAC6 constructs as indicated were assessed for the uptake of dextran (70 kDa)-Texas Red as described for panel C. Values are averages plus standard errors of the means from four independent experiments. *, t < 0.05 in two-tailed and paired t test. (E) Transferrin uptake remained unaffected in HDAC6 KO MEFs. Biotinylated transferrin was incubated with wild-type or KO MEFs, and internalized transferrin at different time points was measured as described in Materials and Methods. Average values from two independent experiments with quadruple wells are shown.
FIG. 5.
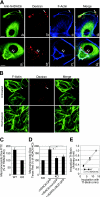
Hsp90 regulates membrane ruffle formation and cell migration. (A) Colocalization of Hsp90 (b, e, and h) with HDAC6 (a) or F-actin (d and g) in wild-type MEFs stimulated with 50 ng/ml PDGF-BB for 8 min. Filled arrowheads indicate ruffles. Open arrowheads in d to f point to Hsp90 (red)-containing F-actin (green) spots. Open arrowheads in g to i indicate colocalization of Hsp90 (red) and F-actin (green) at the macropinosomes. Bar = 10 μm. (B) Inhibition of PDGF-induced dorsal ruffle formation in wild-type MEFs was dose responsive to geldanamycin treatment (2 h at 3 μM or 10 μM). Bars represent average values plus standard errors of the means; n = 4. DMSO, dimethyl sulfoxide (C) Cells pretreated with either vehicle or geldanamycin at 1 μM or 5 μM for 2 h were analyzed for motility. The average value from two independent experiments with duplicate wells is shown. Note that geldanamycin treatment did not induce cytotoxicity under these experimental conditions. (D) Wild-type (WT) and HDAC6 KO MEFs were pretreated with 9 μM geldanamycin (GA) for 2.5 h before they were induced to form ruffles by 50 ng/ml PDGF-BB (8 min at 37°C). Cells are stained with phalloidin-rhodamine for F-actin. At least 500 cells of each cell type were examined. Cells forming dorsal ruffles were scored. Average values plus standard errors of the means from four independent experiments are graphed. (E) HDAC6 KO MEFs were transfected with the FLAG-tagged wild type, mutant human Hsp90α constructs, or a control plasmid as indicated. Cells were stimulated with 50 ng/ml PDGF-BB and were stained with phalloidin-rhodamine for membrane ruffles and anti-FLAG to identify FLAG-tagged Hsp90α-expressing cells, respectively. Cells that formed dorsal ruffles were scored (average value plus standard error). At least 400 FLAG-positive and 800 nontransfected cells on the same cover glass were examined in four independent experiments. The dotted line indicates the level of ruffle formation in nontransfected cells. **, t < 0.01 in a two-tailed t test.
FIG. 6.
Functional HDAC6 and Hsp90 are required for efficient Rac1 activation. (A) Wild-type MEFs were stimulated with 50 ng/ml PDGF-BB for 8 min. Colocalization of Rac1 (b) with F-actin (a) was observed at dorsal ruffle (filled arrowheads) and F-actin dots (open arrowheads) using anti-Rac1 antibody and phalloidin-Alexa Fluor 488. Bar = 10 μm. (B) Rac1 activation in wild-type (WT) and HDAC6 KO MEFs was evaluated by GST-PAK1-CRIB pull-down assay. A diminished Rac1 activation in response to PDGF-BB treatment was observed in HDAC6 KO MEFs. (C) Pretreatment of wild-type MEFs with vehicle control, wortmannin (Wort) (100 nM for 30 min as another control), or geldanamycin at indicated concentrations for 2 h led to reduced Rac1 activation by PDGF-BB treatment (50 ng/ml for 10 min) in a GST-PAK1-CRIB pull-down assay. Note that wortmannin treatment inhibits PI3K function and Rac1 activation (23, 44). DMSO, dimethyl sulfoxide.
Similar articles
- The microtubule-associated histone deacetylase 6 (HDAC6) regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation.
Gao YS, Hubbert CC, Yao TP. Gao YS, et al. J Biol Chem. 2010 Apr 9;285(15):11219-26. doi: 10.1074/jbc.M109.042754. Epub 2010 Feb 4. J Biol Chem. 2010. PMID: 20133936 Free PMC article. - Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors.
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K. Bali P, et al. J Biol Chem. 2005 Jul 22;280(29):26729-34. doi: 10.1074/jbc.C500186200. Epub 2005 Jun 2. J Biol Chem. 2005. PMID: 15937340 - Extracellular signal-regulated kinase (ERK) phosphorylates histone deacetylase 6 (HDAC6) at serine 1035 to stimulate cell migration.
Williams KA, Zhang M, Xiang S, Hu C, Wu JY, Zhang S, Ryan M, Cox AD, Der CJ, Fang B, Koomen J, Haura E, Bepler G, Nicosia SV, Matthias P, Wang C, Bai W, Zhang X. Williams KA, et al. J Biol Chem. 2013 Nov 15;288(46):33156-70. doi: 10.1074/jbc.M113.472506. Epub 2013 Oct 2. J Biol Chem. 2013. PMID: 24089523 Free PMC article. - Posttranslational modification and beyond: interplay between histone deacetylase 6 and heat-shock protein 90.
Liu P, Xiao J, Wang Y, Song X, Huang L, Ren Z, Kitazato K, Wang Y. Liu P, et al. Mol Med. 2021 Sep 16;27(1):110. doi: 10.1186/s10020-021-00375-3. Mol Med. 2021. PMID: 34530730 Free PMC article. Review. - HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions.
Valenzuela-Fernández A, Cabrero JR, Serrador JM, Sánchez-Madrid F. Valenzuela-Fernández A, et al. Trends Cell Biol. 2008 Jun;18(6):291-7. doi: 10.1016/j.tcb.2008.04.003. Epub 2008 May 9. Trends Cell Biol. 2008. PMID: 18472263 Review.
Cited by
- Zinc-Dependent Histone Deacetylases in Lung Endothelial Pathobiology.
Patil RS, Maloney ME, Lucas R, Fulton DJR, Patel V, Bagi Z, Kovacs-Kasa A, Kovacs L, Su Y, Verin AD. Patil RS, et al. Biomolecules. 2024 Jan 23;14(2):140. doi: 10.3390/biom14020140. Biomolecules. 2024. PMID: 38397377 Free PMC article. Review. - HDAC6 regulates mutant SOD1 aggregation through two SMIR motifs and tubulin acetylation.
Gal J, Chen J, Barnett KR, Yang L, Brumley E, Zhu H. Gal J, et al. J Biol Chem. 2013 May 24;288(21):15035-45. doi: 10.1074/jbc.M112.431957. Epub 2013 Apr 11. J Biol Chem. 2013. PMID: 23580651 Free PMC article. - Microtubule destabilization caused by particulate matter contributes to lung endothelial barrier dysfunction and inflammation.
Karki P, Meliton A, Sitikov A, Tian Y, Ohmura T, Birukova AA. Karki P, et al. Cell Signal. 2019 Jan;53:246-255. doi: 10.1016/j.cellsig.2018.10.010. Epub 2018 Oct 16. Cell Signal. 2019. PMID: 30339829 Free PMC article. - The microtubule-associated histone deacetylase 6 (HDAC6) regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation.
Gao YS, Hubbert CC, Yao TP. Gao YS, et al. J Biol Chem. 2010 Apr 9;285(15):11219-26. doi: 10.1074/jbc.M109.042754. Epub 2010 Feb 4. J Biol Chem. 2010. PMID: 20133936 Free PMC article. - HDAC6 Inhibition Extinguishes Autophagy in Cancer: Recent Insights.
Passaro E, Papulino C, Chianese U, Toraldo A, Congi R, Del Gaudio N, Nicoletti MM, Benedetti R, Altucci L. Passaro E, et al. Cancers (Basel). 2021 Dec 14;13(24):6280. doi: 10.3390/cancers13246280. Cancers (Basel). 2021. PMID: 34944907 Free PMC article. Review.
References
- Bali, P., M. Pranpat, J. Bradner, M. Balasis, W. Fiskus, F. Guo, K. Rocha, S. Kumaraswamy, S. Boyapalle, P. Atadja, E. Seto, and K. Bhalla. 2005. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 280:26729-26734. - PubMed
- Barker, S. A., K. K. Caldwell, A. Hall, A. M. Martinez, J. R. Pfeiffer, J. M. Oliver, and B. S. Wilson. 1995. Wortmannin blocks lipid and protein kinase activities associated with PI 3-kinase and inhibits a subset of responses induced by Fc epsilon R1 cross-linking. Mol. Biol. Cell 6:1145-1158. - PMC - PubMed
- Bar-Sagi, D., and A. Hall. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227-238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases