Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse - PubMed (original) (raw)
Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse
S M Berman et al. Mol Psychiatry. 2008 Sep.
Abstract
Changes in brain function during the initial weeks of abstinence from chronic methamphetamine abuse may substantially affect clinical outcome, but are not well understood. We used positron emission tomography with [F-18]fluorodeoxyglucose (FDG) to quantify regional cerebral glucose metabolism, an index of brain function, during performance of a vigilance task. A total of 10 methamphetamine-dependent subjects were tested after 5-9 days of abstinence, and after 4 additional weeks of supervised abstinence. A total of 12 healthy control subjects were tested at corresponding times. Global glucose metabolism increased between tests (P=0.01), more in methamphetamine-dependent (10.9%, P=0.02) than control subjects (1.9%, NS). Glucose metabolism did not change in subcortical regions of methamphetamine-dependent subjects, but increased in neocortex, with maximal increase (>20%) in parietal regions. Changes in reaction time and self-reports of negative affect varied more in methamphetamine-dependent than in control subjects, and correlated both with the increase in parietal glucose metabolism, and decrease in relative activity (after scaling to the global mean) in some regions. A robust relationship between change in self-reports of depressive symptoms and relative activity in the ventral striatum may have great relevance to treatment success because of the role of this region in drug abuse-related behaviors. Shifts in cortical-subcortical metabolic balance either reflect new processes that occur during early abstinence, or the unmasking of effects of chronic methamphetamine abuse that are obscured by suppression of cortical glucose metabolism that continues for at least 5-9 days after cessation of methamphetamine self-administration.
Figures
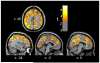
Figure 1. Parietal cortex CMRglc increased between sessions in the MA abuse group, more than in the control group
The figures are depicted in neurological orientation. The gray-scale image is a T1 structural MRI that is representative of MNI space, where positive values of the x, y, and z coordinates approximately represent mm to the right, anterior and superior relative to the sagittal midpoint of the anterior commissure. The a priori parietal lobe regions of interest (ROIs) are outlined in light blue. Red/yellow colors indicate greater increases in CMRglc from initial PET session to four weeks later in 9 MA, as compared to 7 control subjects (p < .01 uncorrected).
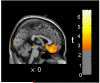
Figure 2. Between-session directional change in relative radioactivity of medial orbitofrontal cortex was inversely correlated with vigilance reaction time (RT) in the MA abuse group
Red/yellow colors indicate areas where the amount of RT slowing from initial PET session to four weeks later was associated with decreased regional relative radioactivity in 10 MA subjects (p < .01 uncorrected).
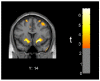
Figure 3. Between-session directional change in relative radioactivity of ventral striatum was directly correlated with self-reported depressive symptoms in the MA abuse group
Red/yellow colors indicate areas where change in BDI score from initial PET session to four weeks later was associated with change in regional relative radioactivity in 10 MA subjects (p < .01 uncorrected).
Similar articles
- Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers.
London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. London ED, et al. Arch Gen Psychiatry. 2004 Jan;61(1):73-84. doi: 10.1001/archpsyc.61.1.73. Arch Gen Psychiatry. 2004. PMID: 14706946 - Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers.
London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. London ED, et al. Biol Psychiatry. 2005 Nov 15;58(10):770-8. doi: 10.1016/j.biopsych.2005.04.039. Epub 2005 Aug 10. Biol Psychiatry. 2005. PMID: 16095568 - Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers.
Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, Logan J. Volkow ND, et al. Am J Psychiatry. 2001 Mar;158(3):383-9. doi: 10.1176/appi.ajp.158.3.383. Am J Psychiatry. 2001. PMID: 11229978 - Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: Is VMAT2 a stable dopamine neuron biomarker?
Boileau I, Rusjan P, Houle S, Wilkins D, Tong J, Selby P, Guttman M, Saint-Cyr JA, Wilson AA, Kish SJ. Boileau I, et al. J Neurosci. 2008 Sep 24;28(39):9850-6. doi: 10.1523/JNEUROSCI.3008-08.2008. J Neurosci. 2008. PMID: 18815269 Free PMC article. - Structural and metabolic brain changes in the striatum associated with methamphetamine abuse.
Chang L, Alicata D, Ernst T, Volkow N. Chang L, et al. Addiction. 2007 Apr;102 Suppl 1:16-32. doi: 10.1111/j.1360-0443.2006.01782.x. Addiction. 2007. PMID: 17493050 Review.
Cited by
- Denial in methamphetamine users: Associations with cognition and functional connectivity in brain.
Dean AC, Kohno M, Morales AM, Ghahremani DG, London ED. Dean AC, et al. Drug Alcohol Depend. 2015 Jun 1;151:84-91. doi: 10.1016/j.drugalcdep.2015.03.004. Epub 2015 Mar 18. Drug Alcohol Depend. 2015. PMID: 25840750 Free PMC article. - Educational attainment is not a good proxy for cognitive function in methamphetamine dependence.
Dean AC, Hellemann G, Sugar CA, London ED. Dean AC, et al. Drug Alcohol Depend. 2012 Jun 1;123(1-3):249-54. doi: 10.1016/j.drugalcdep.2011.11.019. Epub 2011 Dec 27. Drug Alcohol Depend. 2012. PMID: 22206606 Free PMC article. - A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells.
Maoz BM, Herland A, FitzGerald EA, Grevesse T, Vidoudez C, Pacheco AR, Sheehy SP, Park TE, Dauth S, Mannix R, Budnik N, Shores K, Cho A, Nawroth JC, Segrè D, Budnik B, Ingber DE, Parker KK. Maoz BM, et al. Nat Biotechnol. 2018 Oct;36(9):865-874. doi: 10.1038/nbt.4226. Epub 2018 Aug 20. Nat Biotechnol. 2018. PMID: 30125269 Free PMC article. - EANM procedure guidelines for brain PET imaging using [18F]FDG, version 3.
Guedj E, Varrone A, Boellaard R, Albert NL, Barthel H, van Berckel B, Brendel M, Cecchin D, Ekmekcioglu O, Garibotto V, Lammertsma AA, Law I, Peñuelas I, Semah F, Traub-Weidinger T, van de Giessen E, Van Weehaeghe D, Morbelli S. Guedj E, et al. Eur J Nucl Med Mol Imaging. 2022 Jan;49(2):632-651. doi: 10.1007/s00259-021-05603-w. Epub 2021 Dec 9. Eur J Nucl Med Mol Imaging. 2022. PMID: 34882261 Free PMC article. - Neuropathology of substance use disorders.
Cadet JL, Bisagno V, Milroy CM. Cadet JL, et al. Acta Neuropathol. 2014 Jan;127(1):91-107. doi: 10.1007/s00401-013-1221-7. Epub 2013 Nov 29. Acta Neuropathol. 2014. PMID: 24292887 Free PMC article. Review.
References
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch. Gen. Psychiatry. 2004;61:73–84. - PubMed
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol. Psychiatry. 2003;53:65–74. - PubMed
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. - PubMed
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. Neurophysiology Clin. 2003;15:215–220. - PubMed
- Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, et al. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res. 2002;111:65–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA020726-03/DA/NIDA NIH HHS/United States
- R01 DA020726-03S1/DA/NIDA NIH HHS/United States
- M01 RR000865-338778/RR/NCRR NIH HHS/United States
- R01 DA020726-02/DA/NIDA NIH HHS/United States
- MOI RR 00865/RR/NCRR NIH HHS/United States
- M01 RR000865-358126/RR/NCRR NIH HHS/United States
- 1 Y01 DA 50038/DA/NIDA NIH HHS/United States
- M01 RR000865-366865/RR/NCRR NIH HHS/United States
- R01 DA015179-03/DA/NIDA NIH HHS/United States
- R01 DA020726-04/DA/NIDA NIH HHS/United States
- R21 DA050038/DA/NIDA NIH HHS/United States
- R01 DA015179-02/DA/NIDA NIH HHS/United States
- M01 RR000865-328484/RR/NCRR NIH HHS/United States
- R01 DA020726/DA/NIDA NIH HHS/United States
- R01 DA015179/DA/NIDA NIH HHS/United States
- M01 RR000865/RR/NCRR NIH HHS/United States
- R01 DA020726-01/DA/NIDA NIH HHS/United States
- M01 RR000865-366842/RR/NCRR NIH HHS/United States
- M01 RR000865-348735/RR/NCRR NIH HHS/United States
- 5R01 DA 020726/DA/NIDA NIH HHS/United States
- M01 RR000865-348678/RR/NCRR NIH HHS/United States
- 5R01 DA 15179/DA/NIDA NIH HHS/United States
- R01 DA015179-02S1/DA/NIDA NIH HHS/United States
- R01 DA015179-01A1/DA/NIDA NIH HHS/United States
- M01 RR000865-358084/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous