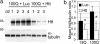Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits - PubMed (original) (raw)
. 2007 Oct 23;104(43):17204-9.
doi: 10.1073/pnas.0708285104. Epub 2007 Oct 16.
M Sena-Esteves, K Chase, E Sapp, E Pfister, M Sass, J Yoder, P Reeves, R K Pandey, K G Rajeev, M Manoharan, D W Y Sah, P D Zamore, N Aronin
Affiliations
- PMID: 17940007
- PMCID: PMC2040405
- DOI: 10.1073/pnas.0708285104
Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits
M DiFiglia et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Huntington's disease (HD) is a neurodegenerative disorder caused by expansion of a CAG repeat in the huntingtin (Htt) gene. HD is autosomal dominant and, in theory, amenable to therapeutic RNA silencing. We introduced cholesterol-conjugated small interfering RNA duplexes (cc-siRNA) targeting human Htt mRNA (siRNA-Htt) into mouse striata that also received adeno-associated virus containing either expanded (100 CAG) or wild-type (18 CAG) Htt cDNA encoding huntingtin (Htt) 1-400. Adeno-associated virus delivery to striatum and overlying cortex of the mutant Htt gene, but not the wild type, produced neuropathology and motor deficits. Treatment with cc-siRNA-Htt in mice with mutant Htt prolonged survival of striatal neurons, reduced neuropil aggregates, diminished inclusion size, and lowered the frequency of clasping and footslips on balance beam. cc-siRNA-Htt was designed to target human wild-type and mutant Htt and decreased levels of both in the striatum. Our findings indicate that a single administration into the adult striatum of an siRNA targeting Htt can silence mutant Htt, attenuate neuronal pathology, and delay the abnormal behavioral phenotype observed in a rapid-onset, viral transgenic mouse model of HD.
Conflict of interest statement
Conflict of interest statement: P.D.Z. is a cofounder and scientific advisory board member of Alnylam Pharmaceuticals. D.W.Y.S., M.M., K.G.R., and R.K.P. are employees of Alnylam Pharmaceuticals.
Figures
Fig. 1.
Cholesterol-conjugated and unconjugated siRNA-Htt enters neurons. (a and b) Laser confocal images of DARPP32-labeled striatal neurons (green) treated with Cy3-siRNA-Htt. The striatum was injected with 10 μM unconjugated (a) or cc (b) Cy3-siRNA-Htt. Cy3 fluorescence appears in the neuronal cytoplasm as distinct bodies (red-orange color at arrows). Images were from striata 5 days (a) and 1 day (b) after injection. (c and d) Cy3 fluorescence in the striatal neuropil surrounding the DARPP32-labeled neurons. (e) Percent of mice with high Cy3 fluorescence in neuropil after injection of cc-siRNA-Htt (n = 17) or unconjugated siRNA-Htt (n = 18); not significant in Fisher's exact test. Bars indicate the confidence intervals.
Fig. 2.
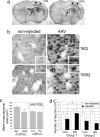
Cholesterol-conjugated siRNA-Htt reversed neuropathology in the AAV HD mouse model. (a) Brain sections from normal mice that received a unilateral striatal injection of AAV_Htt_18Q (Left) or AAV_Htt_100Q (Right). Sections were immunoperoxidase-labeled by using anti-Htt antisera. Exogenous human wild-type and mutant Htt expression is visible in dorsal striatum (asterisks), septal nuclei (arrows), and deep layers of the cortex (arrowheads). (b) Htt-labeled striatal cells infected with AAV_Htt_100Q are smaller than cells expressing Htt18Q or neurons in the noninjected striatum. Insets show examples of Htt-labeled cells at higher magnification. (c) Mean ± SD for the cross-sectional area of immunoreactive striatal neurons in mice infected with AAV_Htt_18Q or AAV_Htt_100Q (Left) and mice infected with AAV_Htt_100Q and cotreated with siRNA-Luc (Luc) or siRNA-Htt (Htt) (Right). Neuronal size is reduced in striatal cells expressing mutant Htt100Q compared with striatal cells expressing Htt18Q (Left; *, P ≤ 0.003; n = 8 mice per group; 50 cells per mouse; Student's t test) but is not changed by cotreatment with cc-siRNA-Htt (Right; n = 4 per group). (d) Number of neurons determined by stereology in Nissl-stained sections in noninjected and AAV_Htt_100Q-injected striatum. Bar graphs show mean ± SD for number of neurons. Group 1 (Left): *, P ≤ 0.01, Student's t test. Group 2 (Right): not significant.
Fig. 3.
Inclusion pathology was reduced in AAV_Htt_100Q mice treated with cc-siRNA-Htt. Immunoperoxidase labeling was done with anti-Htt antisera. (a and b) Neurons with AAV_Htt_100Q inclusions in striatum and cortex. (c) Nuclear inclusions in striatal neurons labeled with EM48 antisera. (d) Scatter plot showing size distribution of inclusions in the two groups of mice treated with AAV_Htt_100Q. Densitometry was performed with EM48-stained sections. Horizontal bars indicate the medians. cc-siRNA-Htt (Htt) treatment reduces median inclusion size compared with cc-siRNA-Luc (Luc). Group 1: n = 8, 8, 8, 8; cortex, P ≤ 0.01; striatum, P ≤ 0.01. Group 2: n = 5, 6, 5, 6. Cortex, not significant; striatum, P ≤ 0.004. One hundred cells per mouse were evaluated. Mann–Whitney U test; *, P ≤ 0.01 for cortex and striatum. (e) Density of Htt-labeled neurons with inclusions per 2,500 μm2. cc-siRNA-Htt treatment increased the number of Htt-labeled neurons with inclusions. Findings are significant for striatum (*, P ≤ 0.002; Student's t test; cc-siRNA-Luc, n = 9; cc-siRNA-Htt, n = 8).
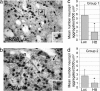
Fig. 4.
Mutant Htt neuropil aggregates were reduced by cc-siRNA-Htt. (a and b) Htt labeling in neuropil aggregates (arrows) in the striatum from mice treated with AAV_Htt_100Q and cc-siRNA-Luc (a) or cc-siRNA-Htt (b). The aggregates are small structures (a), which are faintly visible in b. The boxes in a and b denote the brain region in the Insets. (c and d) Bar graphs showing number of neuropil aggregates in group 1 and group 2 AAV_Htt_100Q mice treated with cc-siRNA-Luc (Luc) or cc-siRNA-Htt (Htt). Group 1: n = 8 per treatment; *, P ≤ 0.002. Group 2: cc-siRNA-Luc, n = 5; cc-siRNA-Htt, n = 6; *, P ≤ 0.036. Student's t test; one ×40 field per mouse.
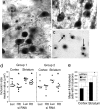
Fig. 5.
AAV_Htt_100Q mice show reduced motor deficits in the presence of cc-siRNA-Htt. (a) Percent of mice clasping 14 days after injection with AAV_Htt_18Q and AAV_Htt_100Q. Mice with AAV_Htt_100Q had significantly more clasping days than mice infected with AAV_Htt_18Q. AAV_Htt_18Q, 15% clasping, n = 13; AAV_Htt_100Q, 62.5% clasping, n = 16 (P = 0.02). (b) AAV_Htt_100Q mice cotreated with cc-siRNA-Htt had fewer clasping days than mice coinjected with cc-siRNA-Luc (group 1: cc-siRNA-Luc, 33%, n = 9, versus cc-siRNA-Htt, 18%, n = 8; *, P = 0.01; group 2: cc-siRNA-Luc, 75%, n = 5, versus cc-siRNA-Htt, 52%, n = 6; *, P ≤ 0.02; Fisher's exact test). (c) Shown are mean ± SD footslips that occurred during beam walking for mice injected with AAV_Htt_100Q. Mean footslips were reduced in the presence of cc-siRNA-Htt compared with cc-siRNA-Luc. *, P ≤ 0.01; Student's t test; n = 4 per group.
Fig. 6.
Silencing human Htt mRNA reduced amount of exogenous wild-type and mutant Htt in the striatum. (a) Lysates were prepared from dorsal striatum 2.7 days after coinjection of AAV_Htt_18Q or AAV_Htt_100Q with either cc-siRNA-Luc or cc-siRNA-Htt. The Western blot was probed first with anti-Htt antibody (Upper) and then reprobed with anti-tubulin antibody (Lower). Shown is expression of Htt100Q in mice treated with cc-siRNA-Luc or cc-siRNA-Htt. (b) Bar graphs show mean Htt/tubulin ratios from densitometry of Western blot films for mice infected with AAV_Htt_18Q (n = 4) or AAV_Htt_100Q (n = 3) and treated with cc-siRNA-Luc or cc-siRNA-Htt. *, P = 0.03 for AAV_Htt_18Q; *, P = 0.01 for AAV_Htt_100Q.
Similar articles
- Silencing mutant huntingtin by adeno-associated virus-mediated RNA interference ameliorates disease manifestations in the YAC128 mouse model of Huntington's disease.
Stanek LM, Sardi SP, Mastis B, Richards AR, Treleaven CM, Taksir T, Misra K, Cheng SH, Shihabuddin LS. Stanek LM, et al. Hum Gene Ther. 2014 May;25(5):461-74. doi: 10.1089/hum.2013.200. Epub 2014 Mar 21. Hum Gene Ther. 2014. PMID: 24484067 Free PMC article. - mRNA Nuclear Clustering Leads to a Difference in Mutant Huntingtin mRNA and Protein Silencing by siRNAs In Vivo.
Allen S, O'Reilly D, Miller R, Sapp E, Summers A, Paquette J, Echeverria Moreno D, Bramato B, McHugh N, Yamada K, Aronin N, DiFiglia M, Khvorova A. Allen S, et al. Nucleic Acid Ther. 2024 Aug;34(4):164-172. doi: 10.1089/nat.2024.0027. Epub 2024 Jul 18. Nucleic Acid Ther. 2024. PMID: 39023561 - Clinico-pathological rescue of a model mouse of Huntington's disease by siRNA.
Wang YL, Liu W, Wada E, Murata M, Wada K, Kanazawa I. Wang YL, et al. Neurosci Res. 2005 Nov;53(3):241-9. doi: 10.1016/j.neures.2005.06.021. Epub 2005 Aug 10. Neurosci Res. 2005. PMID: 16095740 - [Gene silencing approaches for the treatment of Huntington's disease].
Merienne N, Déglon N. Merienne N, et al. Med Sci (Paris). 2015 Feb;31(2):159-67. doi: 10.1051/medsci/20153102012. Epub 2015 Mar 4. Med Sci (Paris). 2015. PMID: 25744262 Review. French. - Translation of MicroRNA-Based Huntingtin-Lowering Therapies from Preclinical Studies to the Clinic.
Miniarikova J, Evers MM, Konstantinova P. Miniarikova J, et al. Mol Ther. 2018 Apr 4;26(4):947-962. doi: 10.1016/j.ymthe.2018.02.002. Epub 2018 Feb 8. Mol Ther. 2018. PMID: 29503201 Free PMC article. Review.
Cited by
- siRNA Therapeutics: Future Promise for Neurodegenerative Diseases.
Amiri A, Barreto G, Sathyapalan T, Sahebkar A. Amiri A, et al. Curr Neuropharmacol. 2021;19(11):1896-1911. doi: 10.2174/1570159X19666210402104054. Curr Neuropharmacol. 2021. PMID: 33797386 Free PMC article. Review. - HSF1 and Its Role in Huntington's Disease Pathology.
Kim H, Gomez-Pastor R. Kim H, et al. Adv Exp Med Biol. 2023;1410:35-95. doi: 10.1007/5584_2022_742. Adv Exp Med Biol. 2023. PMID: 36396925 Review. - Translational research in Huntington's disease: opening up for disease modifying treatment.
Burgunder JM. Burgunder JM. Transl Neurodegener. 2013 Jan 25;2(1):2. doi: 10.1186/2047-9158-2-2. Transl Neurodegener. 2013. PMID: 23347646 Free PMC article. - The role of ALFY in selective autophagy.
Isakson P, Holland P, Simonsen A. Isakson P, et al. Cell Death Differ. 2013 Jan;20(1):12-20. doi: 10.1038/cdd.2012.66. Epub 2012 Jun 1. Cell Death Differ. 2013. PMID: 22653340 Free PMC article. Review. - Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression.
Burgess A, Huang Y, Querbes W, Sah DW, Hynynen K. Burgess A, et al. J Control Release. 2012 Oct 28;163(2):125-9. doi: 10.1016/j.jconrel.2012.08.012. Epub 2012 Aug 19. J Control Release. 2012. PMID: 22921802 Free PMC article.
References
- Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. - PubMed
- Yamamoto A, Lucas JJ, Hen R. Cell. 2000;101:57–66. - PubMed
- Singer O, Marr RA, Rockenstein E, Crews L, Coufal NG, Gage FH, Verma IM, Masliah E. Nat Neurosci. 2005;8:1343–1349. - PubMed
- Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, Henderson CE, Aebischer P. Nat Med. 2005;11:423–428. - PubMed
- Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, Paulson HL, Yang L, Kotin RM, Davidson BL. Nat Med. 2004;10:816–820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases