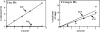MyoD uses overlapping but distinct elements to bind E-box and tetraplex structures of regulatory sequences of muscle-specific genes - PubMed (original) (raw)
MyoD uses overlapping but distinct elements to bind E-box and tetraplex structures of regulatory sequences of muscle-specific genes
Jeny Shklover et al. Nucleic Acids Res. 2007.
Abstract
Muscle differentiation and expression of muscle-specific proteins are initiated by the binding of heterodimers of the transcription factor MyoD with E2A proteins to E-box motif d(CANNTG) in promoters or enhancers of muscle-specific genes. MyoD homodimers, however, form tighter complexes with tetraplex structures of guanine-rich regulatory sequences of some muscle genes. In this work, we identified elements in MyoD that bind E-box or tetraplex structures of promoter sequences of the muscle-specific genes alpha7 integrin and sarcomeric Mitochondrial Creatine Kinase (sMtCK). Deletions of large domains of the 315 amino acids long recombinant MyoD indicated that the binding site for both E-box and tetraplex DNA is its basic region KRKTTNADRRKAATMRERRR that encompasses the three underlined clusters of basic residues designated R(1), R(2) and R(3). Deletion of a single or pairs of R triads or R111C substitution completely abolished the E-box-binding capacity of MyoD. By contrast, the MyoD deletion mutants Delta102-114, DeltaR(3), DeltaR(1)R(3) or DeltaR(2)R(3) maintained comparable tetraplex DNA-binding capacity as reflected by the similar dissociation constants of their protein-DNA complexes. Only deletion of all three basic clusters abolished the binding of tetraplex DNA. Implications of the binding of E-box and tetraplex DNA by non-identical MyoD elements are considered.
Figures
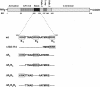
Figure 1.
Scheme of MyoD domains and deletion mutations in its basic region. Deletion mutations were generated within the basic region, (residues 102–121), as described under Materials and Methods section. The triads of basic amino acids, R1, R2 and R3 are boxed.
Figure 2.
Deletion of MyoD residues 102–114 abolishes the binding of E-box but not of G′2 tetraplex integrin DNA. Increasing amounts of full-length MyoD or its Δ102–114 mutant were incubated under binding conditions with 5′-32P labeled double-stranded E-box or G′2 integrin DNA. Protein–DNA complexes were resolved from unbound DNA by non-denaturing polyacrylamide gel electrophoresis and the relative proportions of protein-bound DNA were quantified by phosphor imaging analysis (see Materials and Methods section). Quantification indicated that the bimolecular G′2 integrin DNA was in equilibrium with monomolecular G′4 structure and that MyoD formed complexes solely with the G′2 tetraplex (5). (A) Binding of E-box DNA by full-length and Δ102–114 MyoD. Left—autoradiogram of electrophoretically resolved protein–DNA complexes. Right—plot of the quantified results. (B) Binding of G′2 integrin DNA by full-length and Δ102–114 MyoD. Left—autoradiogram of electrophoretically resolved protein–DNA complexes. Whereas full-length MyoD generated a single protein–DNA complex, the Δ102–114 mutant protein formed two complexes. Right—plot of the quantified results. Percent G′2 integrin DNA bound to Δ102–114 MyoD was the sum of the two types of formed complexes.
Figure 3.
An R111C mutation in MyoD abolishes its E-box-binding activity without affecting the G′2 tetraplex integrin DNA-binding capacity. Full-length or R111C MyoD proteins were bound to 5′-32P labeled E-box or G′2 integrin DNA and protein–DNA complexes were resolved by non-denaturing gel electrophoresis and quantified as detailed in the legend to Figure 2. Presented are plots of percent E-box or G′2 integrin DNA bound as a function of the amounts of added full-length or mutant MyoD.
Figure 4.
Deletion of basic amino acid triads from the MyoD basic region abolishes binding of E-box but not of G′2 tetraplex DNA. The 5′-32P labeled double-stranded E-box or G′2 tetraplex structures of integrin or sMtCK DNA were bound to different amounts of full-length or the indicated mutant MyoD proteins. Protein–DNA complexes were resolved from free DNA by non-denaturing gel electrophoresis as detailed in the legend to Figure 2. (A) Autoradiograms of electrophoretically resolved protein–DNA complexes. Shown are results of DNA binding to 6 and 13 pmol of each examined MyoD protein. (B) Quantified results of the binding of increasing amounts of full-length and mutant MyoD proteins to G′2 tetraplex structures of integrin and sMtCK DNA.
Figure 5.
Representative Scatchard plots of the binding of G′2 integrin DNA to full-length and to ΔR3 mutant MyoD. DNA binding, electrophoretic separation of protein–DNA complexes and their quantification by phosphor imaging were performed as described in the legend to Figure 2. Shown are autoradiograms (insets) and Scatchard plots of the quantified results. The dissociation constants, _K_d, were calculated as detailed under Materials and Methods section.
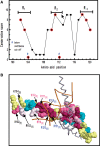
Figure 6.
Residues of the three basic triads are highly conserved. (A) Plot of the conservation score of amino acids that comprise the MyoD basic region. The conservation score of each residue was obtained by the ConSurf 3.0 Web-based program (see Discussion section). Residues of the R1, R2 and R3 basic clusters are marked in red. Scores of residues 104 and 112 were below the confidence cutoff. (B) Conserved residues in the crystal structure of the complex of E-box with the MyoD basic region. Amino acids are color coded in one MyoD monomer according to their conservation score. Residues comprising the basic triads R1 (black font), R2 (red font) and R3 (blue font) are indicated with the conservation score of each logged in parentheses. Also shown are the E-box double helix (orange double ribbon) and the paired MyoD monomer (gray ribbon).
Similar articles
- Homodimeric MyoD preferentially binds tetraplex structures of regulatory sequences of muscle-specific genes.
Etzioni S, Yafe A, Khateb S, Weisman-Shomer P, Bengal E, Fry M. Etzioni S, et al. J Biol Chem. 2005 Jul 22;280(29):26805-12. doi: 10.1074/jbc.M500820200. Epub 2005 May 27. J Biol Chem. 2005. PMID: 15923190 - Formation and properties of hairpin and tetraplex structures of guanine-rich regulatory sequences of muscle-specific genes.
Yafe A, Etzioni S, Weisman-Shomer P, Fry M. Yafe A, et al. Nucleic Acids Res. 2005 May 20;33(9):2887-900. doi: 10.1093/nar/gki606. Print 2005. Nucleic Acids Res. 2005. PMID: 15908587 Free PMC article. - Quadruplex structures of muscle gene promoter sequences enhance in vivo MyoD-dependent gene expression.
Shklover J, Weisman-Shomer P, Yafe A, Fry M. Shklover J, et al. Nucleic Acids Res. 2010 Apr;38(7):2369-77. doi: 10.1093/nar/gkp1208. Epub 2010 Jan 6. Nucleic Acids Res. 2010. PMID: 20053730 Free PMC article. - Differential binding of quadruplex structures of muscle-specific genes regulatory sequences by MyoD, MRF4 and myogenin.
Yafe A, Shklover J, Weisman-Shomer P, Bengal E, Fry M. Yafe A, et al. Nucleic Acids Res. 2008 Jul;36(12):3916-25. doi: 10.1093/nar/gkn340. Epub 2008 May 29. Nucleic Acids Res. 2008. PMID: 18511462 Free PMC article. - Skeletal muscle programming and re-programming.
Fong AP, Tapscott SJ. Fong AP, et al. Curr Opin Genet Dev. 2013 Oct;23(5):568-73. doi: 10.1016/j.gde.2013.05.002. Epub 2013 Jun 4. Curr Opin Genet Dev. 2013. PMID: 23756045 Free PMC article. Review.
Cited by
- Functional studies of the Ciona intestinalis myogenic regulatory factor reveal conserved features of chordate myogenesis.
Izzi SA, Colantuono BJ, Sullivan K, Khare P, Meedel TH. Izzi SA, et al. Dev Biol. 2013 Apr 15;376(2):213-23. doi: 10.1016/j.ydbio.2013.01.033. Epub 2013 Feb 4. Dev Biol. 2013. PMID: 23391688 Free PMC article. - Structures, folding patterns, and functions of intramolecular DNA G-quadruplexes found in eukaryotic promoter regions.
Qin Y, Hurley LH. Qin Y, et al. Biochimie. 2008 Aug;90(8):1149-71. doi: 10.1016/j.biochi.2008.02.020. Epub 2008 Feb 29. Biochimie. 2008. PMID: 18355457 Free PMC article. Review. - MyoD Is a Novel Activator of Porcine FIT1 Gene by Interacting with the Canonical E-Box Element during Myogenesis.
Yan C, Xia X, He J, Ren Z, Xu D, Xiong Y, Zuo B. Yan C, et al. Int J Mol Sci. 2015 Oct 20;16(10):25014-30. doi: 10.3390/ijms161025014. Int J Mol Sci. 2015. PMID: 26492245 Free PMC article. - A novel method for visualizing in-vivo rates of protein degradation provides insight into how TRIM28 regulates muscle size.
Steinert ND, Jorgenson KW, Lin KH, Hermanson JB, Lemens JL, Hornberger TA. Steinert ND, et al. iScience. 2023 Mar 30;26(4):106526. doi: 10.1016/j.isci.2023.106526. eCollection 2023 Apr 21. iScience. 2023. PMID: 37070069 Free PMC article. - Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery.
Asfour HA, Allouh MZ, Said RS. Asfour HA, et al. Exp Biol Med (Maywood). 2018 Jan;243(2):118-128. doi: 10.1177/1535370217749494. Epub 2018 Jan 7. Exp Biol Med (Maywood). 2018. PMID: 29307280 Free PMC article. Review.
References
- Dexheimer T.S., Fry M., Hurley L.H. In: Quadruplex Nucleic Acids. Neidle S., Balasubramanian S., editors. London: RSC Publishing; 2006. pp. 180–207.
- Etzioni S., Yafe A., Khateb S., Weisman-Shomer P., Bengal E., Fry M. Homodimeric MyoD preferentially binds tetraplex structures of regulatory sequences of muscle-specific genes. J. Biol. Chem. 2005;280:26805–26812. - PubMed