Formation and genotoxicity of a guanine-cytosine intrastrand cross-link lesion in vivo - PubMed (original) (raw)
Formation and genotoxicity of a guanine-cytosine intrastrand cross-link lesion in vivo
Haizheng Hong et al. Nucleic Acids Res. 2007.
Abstract
Reactive oxygen species (ROS) can be induced by both endogenous and exogenous processes, and they can damage biological molecules including nucleic acids. Exposure of isolated DNA to X/gamma-rays and Fenton reagents was shown to lead to the formation of intrastrand cross-link lesions where the neighboring nucleobases in the same DNA strand are covalently bonded. By employing HPLC coupled with tandem mass spectrometry (LC-MS/MS) with the isotope dilution method, we assessed quantitatively the formation of a guanine-cytosine (G[8-5]C) intrastrand cross-link lesion in HeLa-S3 cells upon exposure to gamma-rays. The yield of the G[8-5]C cross-link was 0.037 lesions per 10(9) nucleosides per Gy, which was approximately 300 times lower than that of 5-formyl-2'-deoxyuridine (0.011 lesions per 10(6) nucleosides per Gy) under identical exposure conditions. We further constructed a single-stranded M13 genome harboring a site-specifically incorporated G[8-5]C lesion and developed a novel mass spectrometry-based method for interrogating the products emanating from the replication of the genome in Escherichia coli cells. The results demonstrated that G[8-5]C blocked considerably DNA replication as represented by a 20% bypass efficiency, and the lesion was significantly mutagenic in vivo, which included a 8.7% G-->T and a 1.2% G-->C transversion mutations. DNA replication in E. coli hosts deficient in SOS-induced polymerases revealed that polymerase V was responsible for the error-prone translesion synthesis in vivo.
Figures
Figure 1.
Identification of a G[8-5]C intrastrand cross-link lesion in HeLa-S3 cells. Shown are the SICs for the m/z 555→457→261 (a) and m/z 558→460→264 (b) transitions for the in vivo formed intrastrand cross-link and the isotope-labeled internal standard, respectively. Depicted in (c) is the structure for the isotope-labeled internal standard (bold italic letters designate the 15N- and 13C-labeling sites). Panel (d) reveals the dose-responsive formation of the G[8-5]C lesion.
Figure 2.
The REAMS assay for the monitoring of the in vivo cytotoxicity and mutagenicity of the G[8-5]C lesion. For the ligation scaffolds, only the portions of the sequences that are complementary to the 16-mer lesion insert are shown, while the remaining sequences are indicated by black lines. The PCR products and restriction fragments for the competitor genome are not listed. BbsI and Tsp509I cleavage sites are indicated by solid and broken arrows, respectively, and the recognition sites for these two restriction endonucleases are highlighted in bold.
Figure 3.
LC-MS/MS for monitoring the restriction fragments of interest without mutation or with a G→T mutation at the original guanine portion of the lesion [i.e. d(GCGACGCC) and d(TCGACGCC)]. Shown in (a) and (b) are the SICs for the formation of indicated fragment ions of these two ODNs, and illustrated in (c) and (d) are the MS/MS of the [M–2H]2− ions of these two ODNs (m/z 1196.9 and 1184.3). Nomenclature for fragment ions follow that described by McLuckey et al. (56).
Figure 4.
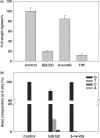
In vivo bypass efficiencies and mutation frequencies of DNA lesions in wild-type AB1157 E. coli cells determined by the REAMS assay. The data represent the means and standard deviations of results from three independent transformation and LC-MS/MS experiments.
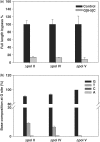
Figure 5.
In vivo bypass efficiencies and mutation frequencies of G[8-5]C in pol II-, pol IV- and pol V-deficient AB1157 E. coli determined by REAMS assay. The data represent the means and standard deviations of results from three independent transformation and LC-MS/MS experiments.
Similar articles
- Identification and quantification of a guanine-thymine intrastrand cross-link lesion induced by Cu(II)/H2O2/ascorbate.
Hong H, Cao H, Wang Y, Wang Y. Hong H, et al. Chem Res Toxicol. 2006 May;19(5):614-21. doi: 10.1021/tx060025x. Chem Res Toxicol. 2006. PMID: 16696563 Free PMC article. - In vivo formation and in vitro replication of a guanine-thymine intrastrand cross-link lesion.
Jiang Y, Hong H, Cao H, Wang Y. Jiang Y, et al. Biochemistry. 2007 Nov 6;46(44):12757-63. doi: 10.1021/bi7012195. Epub 2007 Oct 12. Biochemistry. 2007. PMID: 17929946 - Translesion synthesis in Escherichia coli: lessons from the NarI mutation hot spot.
Fuchs RP, Fujii S. Fuchs RP, et al. DNA Repair (Amst). 2007 Jul 1;6(7):1032-41. doi: 10.1016/j.dnarep.2007.02.021. Epub 2007 Apr 2. DNA Repair (Amst). 2007. PMID: 17403618 Review. - A Comprehensive View of Translesion Synthesis in Escherichia coli.
Fujii S, Fuchs RP. Fujii S, et al. Microbiol Mol Biol Rev. 2020 Jun 17;84(3):e00002-20. doi: 10.1128/MMBR.00002-20. Print 2020 Aug 19. Microbiol Mol Biol Rev. 2020. PMID: 32554755 Free PMC article. Review.
Cited by
- Genetic requirement for mutagenesis of the G[8,5-Me]T cross-link in Escherichia coli: DNA polymerases IV and V compete for error-prone bypass.
Raychaudhury P, Basu AK. Raychaudhury P, et al. Biochemistry. 2011 Mar 29;50(12):2330-8. doi: 10.1021/bi102064z. Epub 2011 Feb 24. Biochemistry. 2011. PMID: 21302943 Free PMC article. - Pyrosequencing: applicability for studying DNA damage-induced mutagenesis.
Minko IG, Earley LF, Larlee KE, Lin YC, Lloyd RS. Minko IG, et al. Environ Mol Mutagen. 2014 Oct;55(8):601-8. doi: 10.1002/em.21882. Epub 2014 Jun 24. Environ Mol Mutagen. 2014. PMID: 24962778 Free PMC article. - Mass Spectrometry-Based Quantitative Strategies for Assessing the Biological Consequences and Repair of DNA Adducts.
You C, Wang Y. You C, et al. Acc Chem Res. 2016 Feb 16;49(2):205-13. doi: 10.1021/acs.accounts.5b00437. Epub 2016 Jan 13. Acc Chem Res. 2016. PMID: 26758048 Free PMC article. Review. - DNA Polymerase II Supports the Replicative Bypass of _N_2-Alkyl-2'-deoxyguanosine Lesions in Escherichia coli Cells.
Wang Y, Wu J, Wu J, Wang Y. Wang Y, et al. Chem Res Toxicol. 2021 Mar 15;34(3):695-698. doi: 10.1021/acs.chemrestox.0c00478. Epub 2021 Jan 8. Chem Res Toxicol. 2021. PMID: 33417436 Free PMC article.
References
- Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. - PubMed
- Lindahl T. DNA lesions generated in vivo by reactive oxygen species, their accumulation and repair. NATO ASI Ser., Ser. A: Life Sci. 1999;302:251–257.
- Marnett L.J. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. - PubMed
- Bellon S., Ravanat J.L., Gasparutto D., Cadet J. Cross-linked thymine-purine base tandem lesions: synthesis, characterization, and measurement in gamma-irradiated isolated DNA. Chem. Res. Toxicol. 2002;15:598–606. - PubMed
- Box H.C., Budzinski E.E., Dawidzik J.B., Gobey J.S., Freund H.G. Free radical-induced tandem base damage in DNA oligomers. Free Radic. Biol. Med. 1997;23:1021–1030. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources