Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture - PubMed (original) (raw)
Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture
R S Talhouk et al. Development. 1991 Jun.
Abstract
The extracellular matrix (ECM) is an important regulator of mammary epithelial cell function both in vivo and in culture. Substantial remodeling of ECM accompanies the structural changes in the mammary gland during gestation, lactation and involution. However, little is known about the nature of the enzymes and the processes involved. We have characterized and studied the regulation of cell-associated and secreted mammary gland proteinases active at neutral pH that may be involved in degradation of the ECM during the different stages of mammary development. Mammary tissue extracts from virgin and pregnant CD-1 mice resolved by zymography contained three major proteinases of 60K (K = 10(3) Mr), 68K and 70K that degraded denatured collagen. These three gelatinases were completely inhibited by the tissue inhibitor of metalloproteinases. Proteolytic activity was lowest during lactation especially for the 60K gelatinase which was shown to be the activated form of the 68K gelatinase. The activated 60K form decreased prior to parturition but increased markedly after the first two days of involution. An additional gelatin-degrading proteinase of 130K was expressed during the first three days of involution and differed from the other gelatinases by its lack of inhibition by the tissue inhibitor of metalloproteinases. The activity of the casein-degrading proteinases was lowest during lactation. Three caseinolytic activities were detected in mammary tissue extracts. A novel 26K cell-associated caseinase--a serine arginine-esterase--was modulated at different stages of mammary development. The other caseinases, at 92K and a larger than 100K, were not developmentally regulated. To find out which cell type produced the proteinases in the mammary gland, we isolated and cultured mouse mammary epithelial cells. Cells cultured on different substrata produced the full spectrum of gelatinases and caseinases seen in the whole gland thus implicating the epithelial cells as a major source of these enzymes. Analysis of proteinases secreted by cells grown on a reconstituted basement membrane showed that gelatinases were secreted preferentially in the direction of the basement membrane. The temporal pattern of expression of these proteinases and the basal secretion of gelatinases by epithelial cells suggest their involvement in the remodelling of the extracellular matrix during the different stages of mammary development and thus modulation of mammary cell function.
Figures
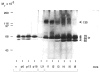
Fig. 1
Gelatinase activity at the various stages of mammary development. Extracts of freshly isolated mammary tissue from 11-to 12-week-old virgin (v), pregnant (p), lactating (L), and involuting (i) CD-1 mice and milk from 9-day lactating mice were assayed for gelatin-degrading activity by SDS–substrate gel zymography. The specific day within each stage of mammary development is indicated in arabic numerals. Lanes were loaded with 15 _μ_g of protein per lane.
Fig. 2
Caseinase activity at the various stages of mammary development. Tissue samples, milk, conditions of the gel and all abbreviations are as described in the legend to Fig. 1.
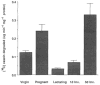
Fig. 3
Quantitative analysis of caseinase activity across mammary gland development. Caseinolytic activity in whole tissue extracts from freshly isolated mammary glands from virgin, mid-pregnant (11–14 days), lactating, 1 day (1d Inv.) and 3 days (3d Inv.) involuting mice, was measured in a soluble [14C]casein-degradation assay. Values are the average caseinase activity of duplicate mammary tissue samples from two mice per stage of development.
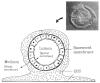
Fig. 4
Schematic diagram of MMEC cultured on EHS-matrix. The cells aggregate into alveolar-like structures with their apical side toward the lumen and their basal side underlined by a basement membrane. Cells in this aggregate retract the EHS-matrix and are engulfed within it. The insert shows a Nomarski image of a thick transverse section of such an alveolus-like structure. With this cell arrangement, the cells secrete basally into the medium (basal compartment) and apically into the lumen (apical compartment). Diagram and inset courtesy of Sally Nash and Charles Streuli respectively.
Fig. 5
Effect of the ECM on the secretion of gelatinases by MMEC in culture. MMEC were grown on EHS-matrix, floating collagen (FG), or plastic under serum-free conditions, and conditioned medium was collected every 24 h and samples from day (d) 4 to 7 were analyzed by SDS–gelatin zymography. Lanes were loaded at equal cell number (5.5×103 cells per lane).
Fig. 6
The distribution of the gelatinases and caseinases into the basal, apical and cellular compartments as resolved on SDS–gel zymograms. Conditioned medium (basal), EGTA-extractable fraction (apical), and cell fraction of MMEC cultured on EHS-matrix in serum-free medium were harvested on day 6 of culture and stored at −70°C. Lanes (basal and apical) were loaded with medium conditioned from 5.5×103 cells. The cell fraction was derived from the same number of cells (5.5×103 cells).
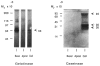
Fig. 7
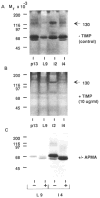
Characterization of the gelatinases. Mammary tissue extracts from 13-day pregnant (pl3), 9-day lactating (L9), and 2- and 4-day involuting (i2 and i4 respectively) mammary gland were separated on SDS–gelatin gels and then incubated without (A) or with (B) TIMP at 10 _μ_g ml−1 in substrate buffer. (C) Activation of the 68K gelatinase after incubation of tissue extract with APMA. L9 and i4 tissue extracts were incubated for 16 h at ambient temperature in 1 mM APMA before loading on the gel. Lanes were loaded with 15 _μ_g of protein.
Fig. 8
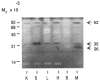
Caseinase activity in different tissues. Extracts of freshly isolated tissue from kidney (K), spleen (S), liver (L), heart (H), brain (B), and mammary gland (M) of 15 day pregnant CD-1 mice were assayed for casein-degrading activity by SDS–substrate gel zymography. Lanes were loaded with 15 _μ_g of protein per lane.
Similar articles
- Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution.
Talhouk RS, Bissell MJ, Werb Z. Talhouk RS, et al. J Cell Biol. 1992 Sep;118(5):1271-82. doi: 10.1083/jcb.118.5.1271. J Cell Biol. 1992. PMID: 1512297 Free PMC article. - Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways.
Lund LR, Rømer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Danø K, Werb Z. Lund LR, et al. Development. 1996 Jan;122(1):181-93. doi: 10.1242/dev.122.1.181. Development. 1996. PMID: 8565829 Free PMC article. - ECM degrading proteases and tissue remodelling in the mammary gland.
Green KA, Lund LR. Green KA, et al. Bioessays. 2005 Sep;27(9):894-903. doi: 10.1002/bies.20281. Bioessays. 2005. PMID: 16108064 Review. - [Mammary gland development: Role of basal myoepithelial cells].
Faraldo MM, Taddei-De La Hosseraye I, Teulière J, Deugnier MA, Moumen M, Thiery JP, Glukhova MA. Faraldo MM, et al. J Soc Biol. 2006;200(2):193-8. doi: 10.1051/jbio:2006021. J Soc Biol. 2006. PMID: 17151555 Review. French.
Cited by
- Changes in the extracellular matrix of the normal human breast during the menstrual cycle.
Ferguson JE, Schor AM, Howell A, Ferguson MW. Ferguson JE, et al. Cell Tissue Res. 1992 Apr;268(1):167-77. doi: 10.1007/BF00338066. Cell Tissue Res. 1992. PMID: 1499048 - Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution.
Talhouk RS, Bissell MJ, Werb Z. Talhouk RS, et al. J Cell Biol. 1992 Sep;118(5):1271-82. doi: 10.1083/jcb.118.5.1271. J Cell Biol. 1992. PMID: 1512297 Free PMC article. - Stromelysin-1 (MMP-3) is a target and a regulator of Wnt1-induced epithelial-mesenchymal transition (EMT).
Blavier L, Lazaryev A, Shi XH, Dorey FJ, Shackleford GM, DeClerck YA. Blavier L, et al. Cancer Biol Ther. 2010 Jul 15;10(2):198-208. doi: 10.4161/cbt.10.2.12193. Epub 2010 Jul 29. Cancer Biol Ther. 2010. PMID: 20534975 Free PMC article. - Breast cancer by proxy: can the microenvironment be both the cause and consequence?
Rønnov-Jessen L, Bissell MJ. Rønnov-Jessen L, et al. Trends Mol Med. 2009 Jan;15(1):5-13. doi: 10.1016/j.molmed.2008.11.001. Epub 2008 Dec 16. Trends Mol Med. 2009. PMID: 19091631 Free PMC article. - A novel pathway for mammary epithelial cell invasion induced by the helix-loop-helix protein Id-1.
Desprez PY, Lin CQ, Thomasset N, Sympson CJ, Bissell MJ, Campisi J. Desprez PY, et al. Mol Cell Biol. 1998 Aug;18(8):4577-88. doi: 10.1128/MCB.18.8.4577. Mol Cell Biol. 1998. PMID: 9671467 Free PMC article.
References
- Adler RR, Brenner CA, Werb Z. Expression of extracellular matrix-degrading metalloproteinases and metalloproteinase inhibitors is developmentally regulated during endoderm differentiation of embryonal carcinoma cells. Development. 1990;110:211–220. - PubMed
- Alexander CM, Werb Z. Proteinases and extracellular matrix remodeling. Current Opinion In Cell Biology. 1989;1:974–982. - PubMed
- Anderson RR. Mammary gland. In: Larson BL, editor. Lactation. Ames, IA: Iowa State University Press; 1985. pp. 3–37.
- Ballin M, Gomez DE, Sinha CC, Thorgeirsson UP. Ras oncogene mediated induction of a 92 kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem biophys Res Commun. 1988;154:832–838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD 23539/HD/NICHD NIH HHS/United States
- U01 CA143233-01/CA/NCI NIH HHS/United States
- U54 CA143836/CA/NCI NIH HHS/United States
- U54 CA112970-01/CA/NCI NIH HHS/United States
- R01 CA064786-05/CA/NCI NIH HHS/United States
- U54 CA126552/CA/NCI NIH HHS/United States
- U54 CA143836-01/CA/NCI NIH HHS/United States
- T32 ES007106/ES/NIEHS NIH HHS/United States
- U54 CA112970/CA/NCI NIH HHS/United States
- U54 CA126552-01/CA/NCI NIH HHS/United States
- R01 CA057621/CA/NCI NIH HHS/United States
- T32ES07106/ES/NIEHS NIH HHS/United States
- U01 CA143233/CA/NCI NIH HHS/United States
- R01 CA057621-07/CA/NCI NIH HHS/United States
- R01 CA064786/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources