Activation of protein tyrosine kinases and matrix metalloproteinases causes blood-brain barrier injury: Novel mechanism for neurodegeneration associated with alcohol abuse - PubMed (original) (raw)
Activation of protein tyrosine kinases and matrix metalloproteinases causes blood-brain barrier injury: Novel mechanism for neurodegeneration associated with alcohol abuse
James Haorah et al. Glia. 2008.
Abstract
Blood-brain barrier (BBB) formed by brain microvascular endothelial cells (BMVEC) regulates the passage of molecules and leukocytes in and out of the brain. Activation of matrix metalloproteinases (MMPs) and alteration of basement membrane (BM) associated with BBB injury was documented in stroke patients. While chronic alcoholism is a risk factor for developing stroke, underlying mechanisms are not well understood. We hypothesized that ethanol (EtOH)-induced protein tyrosine kinase (PTK) signaling resulted a loss of BBB integrity via MMPs activation and degradation of BM component, collagen IV. Treatment of BMVEC with EtOH or acetaldehyde (AA) for 2-48 h increased MMP-1, -2 and -9 activities or decreased the levels of tissue inhibitors of MMPs (TIMP-1, -2) in a PTK-dependent manner without affecting protein tyrosine phosphatase activity. Enhanced PTK activity after EtOH exposure correlated with increased phosphorylated proteins of selective receptor and nonreceptor PTKs. Up-regulation of MMPs activities and protein contents paralleled a decrease in collagen IV content, and inhibitors of EtOH metabolism, MMP-2 and -9, or PTK reversed all these effects. Using human BMVEC assembled into BBB models, we found that EtOH/AA diminished barrier tightness, augmented permeability, and monocyte migration across the BBB via activation of PTKs and MMPs. These findings suggest that alcohol associated BBB injury could be mediated by MMPs via BM protein degradation and could serve as a comorbidity factor for neurological disorders like stroke or neuroinflammation. Furthermore, our preliminary experiments indicated that human astrocytes secreted high levels of MMP-1 and -9 following exposure to EtOH, suggesting the role of BM protein degradation and BBB compromise as a result of glial activation by ethanol. These results provide better understanding of multifaceted effects of alcohol on the brain and could help develop new therapeutic interventions.
(c) 2007 Wiley-Liss, Inc.
Figures
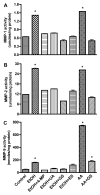
Figure 1
Secreted proteins from BMVEC cultured media after treatment with EtOH 48 hr or with AA for 2 hr were assayed for activity of (A) MMP-1, (B) MMP-2, and (C) MMP-9. Results presented are inducible endogenous active form for MMP-1 and -9, total activity for MMP-2. MMPs activities were calculated from standard curve, and results were expressed as nmoles or μmoles/mg protein (± SEM; n = 4). * indicates p-values <0.01 compared with control. 4-MP (inhibitor of ADH/CYP2E1), UA (antioxidant), GS (PTK inhibitor), ES (inhibitor of MMP-2/-9).
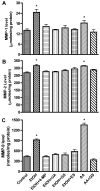
Figure 2
Secreted proteins from BMVEC cultured media after treatment with EtOH 48 hr or with AA for 2 hr were assayed for the content of (A) MMP-1, (B) MMP-2, and (C) MMP-9. Total MMP-1, -2 and -9 protein contents were calculated from standard curve, and results were expressed as ng/μg/ml condition media (± SEM; n = 4). * indicates p-values <0.01 compared with control. Inhibitors did not significantly affect MMPs activity or expression of respective basal controls.
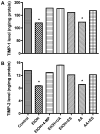
Figure 3
Secreted proteins from BMVEC cultured media after a 48 hr treatment with EtOH or 2 hr with AA were analyzed for levels of (A) TIMP-1 or (B) TIMP-2 expression using the ELISA kits. TIMPs levels were calculated from standard curve and results were expressed as ng/mg protein (± SEM; n = 4). Respective inhibitor did not change the TIMPs expression of basal controls.
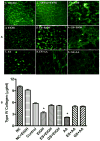
Figure 4
(A) Confocal microscopy analysis of (1) fluorescein-conjugated collagen IV bound to collagen 1 (cell-free), (2) cell-free+EtOH incubated for 72 hr. BMVEC monolayers cultured on top of collagen I and IV layers after 72 hr exposure were (3) control, (4) 50 mM EtOH, (5) 20 μg/ml ES + EtOH, (6) 100 μM GS + EtOH, (7) 100 μM AA, (8) 20 μg/ml ES + AA, (9) 100 μM GS + AA. Original magnification × 600. (B) Degradation of collagen IV by MMPs activation after EtOH/AA stimulation. BMVEC cultured on top of collagen I and IV layers were treated with EtOH/AA for 72 hr followed by quantitative analysis of non-degraded collagen IV amount. Results were expressed as mean μg/ml of undigested collagen IV (± SEM; n = 4).
Figure 5
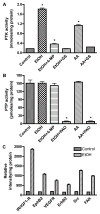
Lysate proteins (20 μg/well) derived from human BMVEC treated with EtOH for 48 hr or with AA for 2 hr were assayed for PTK/PTP activity. Activity of (A) PTK or (B) PTP was calculated from respective standard curve. Results were expressed as mmoles or pmoles/mg protein (± SEM; n = 4). *designates significant decrease (p<0.01) compared to control. PAO is an inhibitor of PTP. (C) Using 100 μg of lysate protein, EtOH-activated receptor and non-receptor PTKs detected by human proteome profiler array kit compared with untreated controls (± SEM; n = 4).
Figure 6
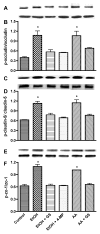
Lysate proteins derived from human BMVEC treated with EtOH for 48 hr or with AA for 2 hr in the presence or absence of 4-MP or GS were subjected to Western blot analysis after immunoprecipitation. Representative immunoreactive bands of (A) occludin-phosphotyrosine, (B) total occludin, (C) ratio of occludin-phosphotyrosine/total occludin, (D) claudin-5-phosphotyrosine, (E) total claudin-5, (F) ratio of claudin-5-phosphotyrosine/total claudin-5, (G) ZO-1-phosphotyrosine, (H) total ZO-1, (I) ratio of ZO-1-phosphotyrosine/total ZO-1. * indicates statistical differences (p < 0.01) compared with control. Respective inhibitor did not change the expression of phosphorylated TJ protein of the basal controls.
Figure 7
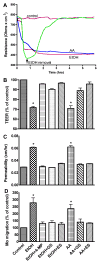
Effects of 50 mM EtOH or 100 μM AA on the BBB functional assays (A) Changes in TEER, (B) 4 kDa dextran permeability, and (C) monocyte migration across the BBB. Prior to TEER, permeability or migration assay, cell monolayers were treated with EtOH for 48 hr or with AA for 2 hr in the presence or absence of specific inhibitor. Results were expressed as percent of controls or apparent permeability coefficient “P” (± SEM; n = 3). Respective inhibitor alone did not change the BBB functional integrity of the basal controls.
Figure 8
Schematic representation of BBB dysfunction due to MMPs activation via PTK signaling pathway stemming from EtOH metabolism in human BMVEC as delayed effects.
Similar articles
- Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction.
Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Haorah J, et al. J Neurochem. 2007 Apr;101(2):566-76. doi: 10.1111/j.1471-4159.2006.04393.x. Epub 2007 Jan 23. J Neurochem. 2007. PMID: 17250680 - Alcohol-induced blood-brain barrier dysfunction is mediated via inositol 1,4,5-triphosphate receptor (IP3R)-gated intracellular calcium release.
Haorah J, Knipe B, Gorantla S, Zheng J, Persidsky Y. Haorah J, et al. J Neurochem. 2007 Jan;100(2):324-36. doi: 10.1111/j.1471-4159.2006.04245.x. J Neurochem. 2007. PMID: 17241155 - Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood-brain barrier compromise.
Haorah J, Heilman D, Knipe B, Chrastil J, Leibhart J, Ghorpade A, Miller DW, Persidsky Y. Haorah J, et al. Alcohol Clin Exp Res. 2005 Jun;29(6):999-1009. doi: 10.1097/01.alc.0000166944.79914.0a. Alcohol Clin Exp Res. 2005. PMID: 15976526 - The role of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the pathophysiology of neurodegeneration: a literature study.
Mroczko B, Groblewska M, Barcikowska M. Mroczko B, et al. J Alzheimers Dis. 2013;37(2):273-83. doi: 10.3233/JAD-130647. J Alzheimers Dis. 2013. PMID: 23792694 Review. - Model systems for studies of leukocyte migration across the blood - brain barrier.
Persidsky Y. Persidsky Y. J Neurovirol. 1999 Dec;5(6):579-90. doi: 10.3109/13550289909021287. J Neurovirol. 1999. PMID: 10602399 Review.
Cited by
- The mechanisms of cerebral vascular dysfunction and neuroinflammation by MMP-mediated degradation of VEGFR-2 in alcohol ingestion.
Muneer PMA, Alikunju S, Szlachetka AM, Haorah J. Muneer PMA, et al. Arterioscler Thromb Vasc Biol. 2012 May;32(5):1167-77. doi: 10.1161/ATVBAHA.112.247668. Epub 2012 Mar 8. Arterioscler Thromb Vasc Biol. 2012. PMID: 22402362 Free PMC article. - Effects of Ethanol on Brain Extracellular Matrix: Implications for Alcohol Use Disorder.
Lasek AW. Lasek AW. Alcohol Clin Exp Res. 2016 Oct;40(10):2030-2042. doi: 10.1111/acer.13200. Epub 2016 Sep 1. Alcohol Clin Exp Res. 2016. PMID: 27581478 Free PMC article. Review. - Function and Mechanism of Myelin Regulation in Alcohol Abuse and Alcoholism.
Rice J, Gu C. Rice J, et al. Bioessays. 2019 Jul;41(7):e1800255. doi: 10.1002/bies.201800255. Epub 2019 May 16. Bioessays. 2019. PMID: 31094014 Free PMC article. Review. - Fetal Alcohol Exposure: The Common Toll.
Nakhoul MR, Seif KE, Haddad N, Haddad GE. Nakhoul MR, et al. J Alcohol Drug Depend. 2017 Feb;5(1):257. doi: 10.4172/2329-6488.1000257. Epub 2017 Feb 28. J Alcohol Drug Depend. 2017. PMID: 28868323 Free PMC article. - Blood-Brain Barrier Disruption and Its Involvement in Neurodevelopmental and Neurodegenerative Disorders.
Aragón-González A, Shaw PJ, Ferraiuolo L. Aragón-González A, et al. Int J Mol Sci. 2022 Dec 3;23(23):15271. doi: 10.3390/ijms232315271. Int J Mol Sci. 2022. PMID: 36499600 Free PMC article. Review.
References
- Aye MM, Ma C, Lin H, Bower KA, Wiggins RC, Luo J. Ethanol-induced in vitro invasion of breast cancer cells: the contribution of MMP-2 by fibroblasts. Int J Cancer. 2004;112(5):738–46. - PubMed
- Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front Biosci. 2007;12:2616–30. - PubMed
- Chen CH, Cheng TH, Lin H, Shih NL, Chen YL, Chen YS, Cheng CF, Lian WS, Meng TC, Chiu WT, et al. Reactive oxygen species generation is involved in epidermal growth factor receptor transactivation through the transient oxidization of Src homology 2-containing tyrosine phosphatase in endothelin-1 signaling pathway in rat cardiac fibroblasts. Mol Pharmacol. 2006;69(4):1347–55. - PubMed
- Didier N, Romero IA, Creminon C, Wijkhuisen A, Grassi J, Mabondzo A. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem. 2003;86(1):246–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH065151/MH/NIMH NIH HHS/United States
- AA017398/AA/NIAAA NIH HHS/United States
- R37 AA015913/AA/NIAAA NIH HHS/United States
- R21 AA013846/AA/NIAAA NIH HHS/United States
- R01 AA015913/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous