Role of RelA of Streptococcus mutans in global control of gene expression - PubMed (original) (raw)
Role of RelA of Streptococcus mutans in global control of gene expression
Marcelle M Nascimento et al. J Bacteriol. 2008 Jan.
Abstract
The production of (p)ppGpp by Streptococcus mutans UA159 is catalyzed by three gene products: RelA, RelP, and RelQ. Here, we investigate the role of the RelA (Rel) homologue of S. mutans in the stringent response and in the global control of gene expression. RelA of S. mutans was shown to synthesize pppGpp in vitro from GTP and ATP in the absence of added ribosomes, as well as in vivo in an Escherichia coli relA-spoT mutant. Mupirocin (MUP) was shown to induce high levels of (p)ppGpp production in S. mutans in a relA-dependent manner, with a concomitant reduction in GTP pools. Transcription profiling after MUP treatment of S. mutans revealed that 104 genes were upregulated and 130 were downregulated (P < or = 0.001); mainly, genes for macromolecular biosynthesis, translation, and energy metabolism were downregulated. When a derivative of UA159 carrying a complete deletion of the relA gene was treated with MUP, 72 genes were upregulated and 52 were downregulated (P < or = 0.001). The expression of 50 genes (P < or = 0.001) was commonly affected by MUP treatment in the two strains, suggesting that S. mutans can mount a relA-independent response to MUP. Consistent with the gene expression profiling, RelA was shown to play major roles in the regulation of phenotypic traits that are required for establishment, persistence, and virulence expression by this oral pathogen. Thus, RelA is the major (p)ppGpp synthase controlling the stringent response in S. mutans, and it coordinates the expression of genes and phenotypes that contribute to the pathogenic potential of the organism.
Figures
FIG. 1.
Complementation of E. coli CF1693 (Δ_relA_ Δ_spoT_) with an MBP fusion of the S. mutans RelA enzyme. (A) Accumulation of (p)ppGpp in E. coli strains after SHx-valine treatment. Strains were labeled with [32P]orthophosphate in MOPS labeling medium. Nucleotides were extracted by adding an equal volume of 13 M formic acid, followed by freeze-thaw cycles. Acid extracts were spotted onto PEI-cellulose plates for TLC in 1.5 M KH2PO4. Lanes: 1, CF1648 (wild type); 2, CF1693 (Δ_relA_ Δ_spoT_); 3, CF1693/pMAL-relA. (B) In vitro (p)ppGpp-synthetic activity of purified S. mutans RelA. Different concentrations (in micrograms as indicated) of purified RelA were incubated at 37°C for 1 h in a reaction mix containing 6 mM [α-32P]GTP and 8 mM ATP as detailed in Materials and Methods. The reaction was terminated by addition of an equal volume of 13 M formic acid, and 5-μl samples were resolved by PEI-TLC in 1.5 M KH2PO4.
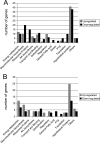
FIG. 2.
Numbers of genes grouped by functional categories that were differently expressed after MUP treatment in comparison to control cells. (A) UA159; (B) JLrelA (Δ_relA_). Gene annotations are based on information provided by the Los Alamos National Laboratory (
) or by published literature available at the same website.
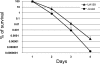
FIG. 3.
Long-term survival of S. mutans UA159 (wild type) and JLrelA (Δ_relA_) in TY medium supplemented with 50 mM glucose. The results represent the means ± the standard deviations of three independent experiments.
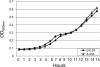
FIG. 4.
Diauxic growth of S. mutans UA159 (wild-type) and JLrelA (Δ_relA_) strains. Mid-exponential-phase cultures grown in BHI were diluted 1:100 in TV medium supplemented with 0.05% glucose and 0.5% inulin, and cell growth was monitored every 1 h. The results represent the means ± the standard deviations of three independent experiments.
Similar articles
- Global regulation by (p)ppGpp and CodY in Streptococcus mutans.
Lemos JA, Nascimento MM, Lin VK, Abranches J, Burne RA. Lemos JA, et al. J Bacteriol. 2008 Aug;190(15):5291-9. doi: 10.1128/JB.00288-08. Epub 2008 Jun 6. J Bacteriol. 2008. PMID: 18539745 Free PMC article. - The Ps and Qs of alarmone synthesis in Staphylococcus aureus.
Yang N, Xie S, Tang NY, Choi MY, Wang Y, Watt RM. Yang N, et al. PLoS One. 2019 Oct 15;14(10):e0213630. doi: 10.1371/journal.pone.0213630. eCollection 2019. PLoS One. 2019. PMID: 31613897 Free PMC article. - Physiological analysis of the stringent response elicited in an extreme thermophilic bacterium, Thermus thermophilus.
Kasai K, Nishizawa T, Takahashi K, Hosaka T, Aoki H, Ochi K. Kasai K, et al. J Bacteriol. 2006 Oct;188(20):7111-22. doi: 10.1128/JB.00574-06. J Bacteriol. 2006. PMID: 17015650 Free PMC article. - Transcriptional organization and physiological contributions of the relQ operon of Streptococcus mutans.
Kim JN, Ahn SJ, Seaton K, Garrett S, Burne RA. Kim JN, et al. J Bacteriol. 2012 Apr;194(8):1968-78. doi: 10.1128/JB.00037-12. Epub 2012 Feb 17. J Bacteriol. 2012. PMID: 22343297 Free PMC article. - Signalling by the global regulatory molecule ppGpp in bacteria and chloroplasts of land plants.
Tozawa Y, Nomura Y. Tozawa Y, et al. Plant Biol (Stuttg). 2011 Sep;13(5):699-709. doi: 10.1111/j.1438-8677.2011.00484.x. Epub 2011 May 31. Plant Biol (Stuttg). 2011. PMID: 21815973 Review.
Cited by
- Many means to a common end: the intricacies of (p)ppGpp metabolism and its control of bacterial homeostasis.
Gaca AO, Colomer-Winter C, Lemos JA. Gaca AO, et al. J Bacteriol. 2015 Apr;197(7):1146-56. doi: 10.1128/JB.02577-14. Epub 2015 Jan 20. J Bacteriol. 2015. PMID: 25605304 Free PMC article. Review. - Peptides encoded in the Streptococcus mutans RcrRPQ operon are essential for thermotolerance.
Shields RC, Kim JN, Ahn SJ, Burne RA. Shields RC, et al. Microbiology (Reading). 2020 Mar;166(3):306-317. doi: 10.1099/mic.0.000887. Microbiology (Reading). 2020. PMID: 31935187 Free PMC article. - Stress Physiology of Lactic Acid Bacteria.
Papadimitriou K, Alegría Á, Bron PA, de Angelis M, Gobbetti M, Kleerebezem M, Lemos JA, Linares DM, Ross P, Stanton C, Turroni F, van Sinderen D, Varmanen P, Ventura M, Zúñiga M, Tsakalidou E, Kok J. Papadimitriou K, et al. Microbiol Mol Biol Rev. 2016 Jul 27;80(3):837-90. doi: 10.1128/MMBR.00076-15. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27466284 Free PMC article. Review. - RelQ-mediated alarmone signalling regulates growth, stress-induced biofilm formation and spore accumulation in Clostridioides difficile.
Malik A, Oludiran A, Poudel A, Alvarez OB, Woodward C, Purcell EB. Malik A, et al. Microbiology (Reading). 2024 Jul;170(7):001479. doi: 10.1099/mic.0.001479. Microbiology (Reading). 2024. PMID: 39028551 Free PMC article. - An Essential Role for (p)ppGpp in the Integration of Stress Tolerance, Peptide Signaling, and Competence Development in Streptococcus mutans.
Kaspar J, Kim JN, Ahn SJ, Burne RA. Kaspar J, et al. Front Microbiol. 2016 Jul 28;7:1162. doi: 10.3389/fmicb.2016.01162. eCollection 2016. Front Microbiol. 2016. PMID: 27516759 Free PMC article.
References
- Balzer, G. J., and R. J. McLean. 2002. The stringent response genes relA and spoT are important for Escherichia coli biofilms under slow-growth conditions. Can. J. Microbiol. 48675-680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases