Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria - PubMed (original) (raw)
. 2007 Oct 29;204(11):2693-704.
doi: 10.1084/jem.20070819. Epub 2007 Oct 22.
Daniel A Lampah, Retno Gitawati, Emiliana Tjitra, Enny Kenangalem, Yvette R McNeil, Christabelle J Darcy, Donald L Granger, J Brice Weinberg, Bert K Lopansri, Ric N Price, Stephen B Duffull, David S Celermajer, Nicholas M Anstey
Affiliations
- PMID: 17954570
- PMCID: PMC2118490
- DOI: 10.1084/jem.20070819
Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria
Tsin W Yeo et al. J Exp Med. 2007.
Abstract
Severe falciparum malaria (SM) is associated with tissue ischemia related to cytoadherence of parasitized erythrocytes to microvascular endothelium and reduced levels of NO and its precursor, l-arginine. Endothelial function has not been characterized in SM but can be improved by l-arginine in cardiovascular disease. In an observational study in Indonesia, we measured endothelial function using reactive hyperemia-peripheral arterial tonometry (RH-PAT) in 51 adults with SM, 48 patients with moderately severe falciparum malaria (MSM), and 48 controls. The mean RH-PAT index was lower in SM (1.41; 95% confidence interval [CI] = 1.33-1.47) than in MSM (1.82; 95% CI = 1.7-2.02) and controls (1.93; 95% CI = 1.8-2.06; P < 0.0001). Endothelial dysfunction was associated with elevated blood lactate and measures of hemolysis. Exhaled NO was also lower in SM relative to MSM and controls. In an ascending dose study of intravenous l-arginine in 30 more patients with MSM, l-arginine increased the RH-PAT index by 19% (95% CI = 6-34; P = 0.006) and exhaled NO by 55% (95% CI = 32-73; P < 0.0001) without important side effects. Hypoargininemia and hemolysis likely reduce NO bioavailability. Endothelial dysfunction in malaria is nearly universal in severe disease, is reversible with l-arginine, and likely contributes to its pathogenesis. Clinical trials in SM of adjunctive agents to improve endothelial NO bioavailability, including l-arginine, are warranted.
Figures
Figure 1.
Study profile of patients recruited in stages 1 and 2 of the study. Stage 1 was an observational study to compare endothelial function among individuals with SM, MSM, and HC. Stage 2 was intervention study to measure the effect of
l
-arginine or saline infusion on RH-PAT index and exhaled NO in MSM.
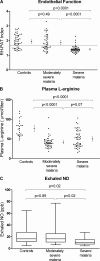
Figure 2.
RH-PAT index, plasma l-arginine concentrations, and exhaled NO in each study group at enrollment. (A) Comparison of RH-PAT index at enrollment among disease categories (P < 0.0001 by ANOVA). Dots and error bars indicate means and 95% CIs. Horizontal line indicates an RH-PAT index of 1.67; values below this represent impaired endothelial function (reference 23). (B) Comparison of plasma
l
-arginine concentrations among disease categories (P < 0.0001 by ANOVA). Dots and bars indicate means and 95% CIs. (C) Comparison of exhaled NO concentrations at enrollment among disease categories (P = 0.049 by the Kruskal-Wallis test). Central line and box indicate the median and interquartile range. Whiskers indicate range.
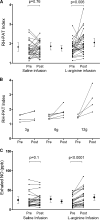
Figure 3.
Change in RH-PAT index and exhaled NO after l-arginine infusion. (A) Change in RH-PAT index in MSM after infusion of 100 ml saline (3%; 95% CI = from −1 to 12) or
l
-arginine (19%; 95% CI = 8–33). p-values refer to paired comparisons before and after infusion. Dots and bars indicate means and 95% CIs. (B) Dose-related change in RH-PAT index after intravenous
l
-arginine infusion in the a priori–defined subset of patients with MSM who had baseline endothelial dysfunction (RH-PAT index <1.67; n = 14; P = 0.03). Lines show RH-PAT index before and after intravenous
l
-arginine at doses of 3, 6, and 12 g. In the 16 patients without baseline impairment of endothelial function (RH-PAT index >1.67), there was no significant change in RH-PAT after
l
-arginine infusion. (C) Change in exhaled NO concentration in MSM after infusion of 100 ml saline (18%; 95% CI = from −2 to 28) or
l
-arginine (55%; 95% CI = 39–71). p-values refer to paired comparisons before and after infusion. Dots and bars indicate means and 95% CIs.
Similar articles
- Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria.
Yeo TW, Lampah DA, Tjitra E, Gitawati R, Kenangalem E, Piera K, Granger DL, Lopansri BK, Weinberg JB, Price RN, Duffull SB, Celermajer DS, Anstey NM. Yeo TW, et al. J Infect Dis. 2009 Nov 15;200(10):1522-9. doi: 10.1086/644641. J Infect Dis. 2009. PMID: 19803726 Free PMC article. - Recovery of endothelial function in severe falciparum malaria: relationship with improvement in plasma L-arginine and blood lactate concentrations.
Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR, Darcy CJ, Granger DL, Weinberg JB, Lopansri BK, Price RN, Duffull SB, Celermajer DS, Anstey NM. Yeo TW, et al. J Infect Dis. 2008 Aug 15;198(4):602-8. doi: 10.1086/590209. J Infect Dis. 2008. PMID: 18605903 Free PMC article. - Pharmacokinetic-Pharmacodynamic Model for the Effect of l-Arginine on Endothelial Function in Patients with Moderately Severe Falciparum Malaria.
Brussee JM, Yeo TW, Lampah DA, Anstey NM, Duffull SB. Brussee JM, et al. Antimicrob Agents Chemother. 2015 Oct 19;60(1):198-205. doi: 10.1128/AAC.01479-15. Print 2016 Jan. Antimicrob Agents Chemother. 2015. PMID: 26482311 Free PMC article. Clinical Trial. - Arginine, nitric oxide, carbon monoxide, and endothelial function in severe malaria.
Weinberg JB, Lopansri BK, Mwaikambo E, Granger DL. Weinberg JB, et al. Curr Opin Infect Dis. 2008 Oct;21(5):468-75. doi: 10.1097/QCO.0b013e32830ef5cf. Curr Opin Infect Dis. 2008. PMID: 18725795 Free PMC article. Review. - Nitric oxide bioavailability in malaria.
Sobolewski P, Gramaglia I, Frangos J, Intaglietta M, van der Heyde HC. Sobolewski P, et al. Trends Parasitol. 2005 Sep;21(9):415-22. doi: 10.1016/j.pt.2005.07.002. Trends Parasitol. 2005. PMID: 16039159 Review.
Cited by
- Increased pulmonary pressures and myocardial wall stress in children with severe malaria.
Janka JJ, Koita OA, Traoré B, Traoré JM, Mzayek F, Sachdev V, Wang X, Sanogo K, Sangaré L, Mendelsohn L, Masur H, Kato GJ, Gladwin MT, Krogstad DJ. Janka JJ, et al. J Infect Dis. 2010 Sep 1;202(5):791-800. doi: 10.1086/655225. J Infect Dis. 2010. PMID: 20662718 Free PMC article. - Loss of complement regulatory proteins on uninfected erythrocytes in vivax and falciparum malaria anemia.
Oyong DA, Kenangalem E, Poespoprodjo JR, Beeson JG, Anstey NM, Price RN, Boyle MJ. Oyong DA, et al. JCI Insight. 2018 Nov 15;3(22):e124854. doi: 10.1172/jci.insight.124854. JCI Insight. 2018. PMID: 30429373 Free PMC article. - Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria.
Yeo TW, Lampah DA, Tjitra E, Gitawati R, Kenangalem E, Piera K, Granger DL, Lopansri BK, Weinberg JB, Price RN, Duffull SB, Celermajer DS, Anstey NM. Yeo TW, et al. J Infect Dis. 2009 Nov 15;200(10):1522-9. doi: 10.1086/644641. J Infect Dis. 2009. PMID: 19803726 Free PMC article. - Arginine metabolism and nutrition in growth, health and disease.
Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y. Wu G, et al. Amino Acids. 2009 May;37(1):153-68. doi: 10.1007/s00726-008-0210-y. Epub 2008 Nov 23. Amino Acids. 2009. PMID: 19030957 Free PMC article. Review. - Recovery of endothelial function in severe falciparum malaria: relationship with improvement in plasma L-arginine and blood lactate concentrations.
Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR, Darcy CJ, Granger DL, Weinberg JB, Lopansri BK, Price RN, Duffull SB, Celermajer DS, Anstey NM. Yeo TW, et al. J Infect Dis. 2008 Aug 15;198(4):602-8. doi: 10.1086/590209. J Infect Dis. 2008. PMID: 18605903 Free PMC article.
References
- The SEAQUAMAT Trial Group. 2005. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 366:717–725. - PubMed
- Pasvol, G. 2005. Management of severe malaria: interventions and controversies. Infect. Dis. Clin. North Am. 19:211–240. - PubMed
- Marchiafava, E., and A. Bignami. 1894. On Summer-Autumnal Fever. London: New Sydenham Society.
- Turner, G.D., H. Morrison, M. Jones, T.M. Davis, S. Looareesuwan, I.D. Buley, K.C. Gatter, C.I. Newbold, S. Pukritayakamee, B. Nagachinta, et al. 1994. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am. J. Pathol. 145:1057–1069. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ICRG GR071614MA/WT_/Wellcome Trust/United Kingdom
- AI041764/AI/NIAID NIH HHS/United States
- R01 AI041764/AI/NIAID NIH HHS/United States
- K23 AI055982/AI/NIAID NIH HHS/United States
- AI55982/AI/NIAID NIH HHS/United States
- R21 AI041764/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources