Cep164, a novel centriole appendage protein required for primary cilium formation - PubMed (original) (raw)
Cep164, a novel centriole appendage protein required for primary cilium formation
Susanne Graser et al. J Cell Biol. 2007.
Abstract
Primary cilia (PC) function as microtubule-based sensory antennae projecting from the surface of many eukaryotic cells. They play important roles in mechano- and chemosensory perception and their dysfunction is implicated in developmental disorders and severe diseases. The basal body that functions in PC assembly is derived from the mature centriole, a component of the centrosome. Through a small interfering RNA screen we found several centrosomal proteins (Ceps) to be involved in PC formation. One newly identified protein, Cep164, was indispensable for PC formation and hence characterized in detail. By immunogold electron microscopy, Cep164 could be localized to the distal appendages of mature centrioles. In contrast to ninein and Cep170, two components of subdistal appendages, Cep164 persisted at centrioles throughout mitosis. Moreover, the localizations of Cep164 and ninein/Cep170 were mutually independent during interphase. These data implicate distal appendages in PC formation and identify Cep164 as an excellent marker for these structures.
Figures
Figure 1.
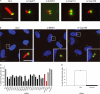
siRNA screen for proteins implicated in PC formation. hTERT-RPE1 cells were transfected for 48 h with control (GL2) or protein-specific oligonucleotide duplexes, followed by serum starvation to induce PC formation. Cells were analyzed by IF microscopy using antibodies against acetylated tubulin (red) and C-Nap1 (green), used to mark cilia and centrioles, respectively. (A) Normal cilia in control cells (GL2) and stunted cilia after siRNA targeting Cep57, ALMS1, Cep131, or Cep152. (B) Normal cilia in control cells (GL2) and suppressed cilia formation after siRNA targeting BBS4 or Cep164. Insets show magnifications of the boxed areas. (C) Influence of various siRNA treatments on the percentage of cells showing PC formation. A reduction in PC formation was considered substantial if siRNA-mediated depletion of a particular centrosomal protein showed a comparable effect as depletion of either pericentrin or Odf2, two proteins previously implicated in PC formation (Jurczyk et al., 2004; Ishikawa et al., 2005). No effects were seen upon siRNA treatments targeting fibroblast growth factor receptor 1 oncogene partner (FOP), rootletin, or C-Nap1. (D) hTERT-RPE1 cells were transfected for 48 h with control (GL2) or Cep164-specific oligonucleotide duplexes. PC formation was monitored specifically in those cells that had been efficiently depleted of Cep164 as determined by staining with anti-Cep164 antibodies. This focus on efficiently depleted cells explains the higher impact of Cep164 depletion on PC formation in these experiments, as compared with the screen (C), in which all cells had been analyzed regardless of siRNA efficiency. At least 250 cells were counted for each bar in four experiments and error bars denote SD. Bars: (A) 5 μm; (B) 10 μm; (B, inset) 5 μm.
Figure 2.
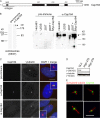
Characterization of anti-Cep164 antibodies. (A) Schematic illustrating primary structure of human Cep164. The protein is predicted to comprise an N-terminal WW domain (WW) and three coiled-coil domains (CC). The horizontal line underneath the scheme denotes the region used for antibody production. (B) Antibodies directed against Cep164 and corresponding preimmune serum were tested by Western blotting on centrosome preparations from KE37 cells (left) and total lysates of HeLaS3, U2OS, and 293T cells as well as 293T cells overexpressing myc-Cep164 (right). Affinity-purified antibody was used at 1 μg/ml. Arrowhead indicates Cep164. Note that in the centrosome sample Cep164 shows a retarded electrophoretic mobility caused by a high sucrose concentration. (C) Antibodies directed against Cep164 and corresponding preimmune serum were used in IF microscopy on U2OS cells. The anti-Cep164 antibody (green) recognizes only one of two centrioles, as indicated by colocalization with _γ_-tubulin (red). This staining was abolished upon siRNA-mediated depletion of Cep164, and preimmune serum showed no specific staining. (insets) Enlarged centrosome areas. (D) Western blot on U2OS cells illustrating efficient depletion of Cep164 by siRNA. Transfections were performed for 48 h using two different Cep164-specific oligonucleotide duplexes (278 and 279) or GL2 for control. Blotting for α-tubulin is shown to control for equal loading. (E) Cep164 localizes to mature centrioles at the base of the PC but not along the length of the axoneme. hTERT-RPE1 cells were serum starved for 48 h to induce PC formation and stained with antibodies against Cep164 (green) and acetylated tubulin (red). The four representative examples show bar- and ringlike staining of Cep164 at the base of the PC in close association with the mature centriole. Bars: (C) 10 μm; (E) 5 μm.
Figure 3.
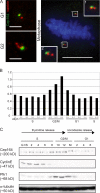
Cell cycle profile of Cep164 expression. (A) U2OS cells were costained with antibodies against Cep164 (green) and centrin as a marker for individual centrioles (red). Note that Cep164 (green) localizes to only one centriole in both G1 centrosomes (top left) and duplicated G2 centrosomes (bottom left). During mitosis, one of the two centrioles present at each spindle pole is positive for Cep164 (right). (insets) Magnifications of the spindle poles. Bars: (interphase cells) 2 μm; (metaphase cell) 10 μm. (B) Cep164 mRNA levels across the cell cycle were determined in synchronized HeLaS3 cells using qRT-PCR. (C) Cep164 protein levels across the cell cycle. HeLaS3 cells were arrested at the G1/S boundary by a double thymidine block or in M phase by a thymidine block followed by nocodazole treatment and released into fresh medium. Samples were harvested at the indicated time points and subjected to immunoblotting using the indicated antibodies.
Figure 4.
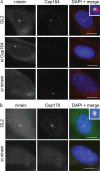
Localizations and mutual dependencies of appendage proteins. (A) U2OS cells were transfected with control (GL2), ninein-specific, or Cep164-specific siRNA duplexes and costained for ninein (red) and Cep164 (green). (inset) Enlarged centrosome area. (B) U2OS cells were transfected with control (GL2) or ninein-specific siRNA duplexes and costained for ninein (red) and Cep170 (green). (inset) Enlarged centrosome area. Bars, 10 μm.
Figure 5.
Immuno-EM localization of Cep164. (A) U2OS cells were subjected to preembedding immuno-EM. Cells were labeled with anti-Cep164 antibody R171 followed by Nanogold-coupled secondary antibody. Note that Cep164 localizes to very distal appendage structures on parental centrioles (arrowheads, also see B), and sometimes electron-dense material (presumably reflecting subdistal appendages) can be seen proximal to the Cep164 staining (arrows in second panel and in B [top]). The schematic diagrams (dotted lines) in the two top panels illustrate the positions of “mother” (MC) and “daughter” centrioles (DC), along with the presumed positions of distal and subdistal appendages. (B and C) U2OS cells were transfected for 48 h with control (GL2) or Cep164-specific siRNA duplexes and subjected to preembedding immuno-EM. Cells were labeled with either anti-Cep164 or anti-ninein antibody followed by Nanogold-coupled secondary antibody. Bars, 250 nm.
Similar articles
- Cep164 triggers ciliogenesis by recruiting Tau tubulin kinase 2 to the mother centriole.
Čajánek L, Nigg EA. Čajánek L, et al. Proc Natl Acad Sci U S A. 2014 Jul 15;111(28):E2841-50. doi: 10.1073/pnas.1401777111. Epub 2014 Jun 30. Proc Natl Acad Sci U S A. 2014. PMID: 24982133 Free PMC article. - A Wnt/beta-catenin pathway antagonist Chibby binds Cenexin at the distal end of mother centrioles and functions in primary cilia formation.
Steere N, Chae V, Burke M, Li FQ, Takemaru K, Kuriyama R. Steere N, et al. PLoS One. 2012;7(7):e41077. doi: 10.1371/journal.pone.0041077. Epub 2012 Jul 20. PLoS One. 2012. PMID: 22911743 Free PMC article. - hVFL3/CCDC61 is a component of mother centriole subdistal appendages required for centrosome cohesion and positioning.
Pizon V, Gaudin N, Poteau M, Cifuentes-Diaz C, Demdou R, Heyer V, Reina San Martin B, Azimzadeh J. Pizon V, et al. Biol Cell. 2020 Jan;112(1):22-37. doi: 10.1111/boc.201900038. Epub 2019 Dec 11. Biol Cell. 2020. PMID: 31789463 - A centriole's subdistal appendages: contributions to cell division, ciliogenesis and differentiation.
Hall NA, Hehnly H. Hall NA, et al. Open Biol. 2021 Feb;11(2):200399. doi: 10.1098/rsob.200399. Epub 2021 Feb 10. Open Biol. 2021. PMID: 33561384 Free PMC article. Review. - Structure and function of distal and subdistal appendages of the mother centriole.
Ma D, Wang F, Teng J, Huang N, Chen J. Ma D, et al. J Cell Sci. 2023 Feb 1;136(3):jcs260560. doi: 10.1242/jcs.260560. Epub 2023 Feb 2. J Cell Sci. 2023. PMID: 36727648 Review.
Cited by
- BAR Domain-Containing FAM92 Proteins Interact with Chibby1 To Facilitate Ciliogenesis.
Li FQ, Chen X, Fisher C, Siller SS, Zelikman K, Kuriyama R, Takemaru KI. Li FQ, et al. Mol Cell Biol. 2016 Oct 13;36(21):2668-2680. doi: 10.1128/MCB.00160-16. Print 2016 Nov 1. Mol Cell Biol. 2016. PMID: 27528616 Free PMC article. - OCRL localizes to the primary cilium: a new role for cilia in Lowe syndrome.
Luo N, West CC, Murga-Zamalloa CA, Sun L, Anderson RM, Wells CD, Weinreb RN, Travers JB, Khanna H, Sun Y. Luo N, et al. Hum Mol Genet. 2012 Aug 1;21(15):3333-44. doi: 10.1093/hmg/dds163. Epub 2012 Apr 27. Hum Mol Genet. 2012. PMID: 22543976 Free PMC article. - A comprehensive analysis of Rab GTPases reveals a role for Rab34 in serum starvation-induced primary ciliogenesis.
Oguchi ME, Okuyama K, Homma Y, Fukuda M. Oguchi ME, et al. J Biol Chem. 2020 Sep 4;295(36):12674-12685. doi: 10.1074/jbc.RA119.012233. Epub 2020 Jul 15. J Biol Chem. 2020. PMID: 32669361 Free PMC article. - A primer on the mouse basal body.
Garcia G 3rd, Reiter JF. Garcia G 3rd, et al. Cilia. 2016 Apr 25;5:17. doi: 10.1186/s13630-016-0038-0. eCollection 2016. Cilia. 2016. PMID: 27114821 Free PMC article. Review. - PLK4 is upregulated in prostate cancer and its inhibition reduces centrosome amplification and causes senescence.
Singh CK, Denu RA, Nihal M, Shabbir M, Garvey DR, Huang W, Iczkowski KA, Ahmad N. Singh CK, et al. Prostate. 2022 Jun;82(9):957-969. doi: 10.1002/pros.24342. Epub 2022 Mar 25. Prostate. 2022. PMID: 35333404 Free PMC article.
References
- Andersen, J.S., C.J. Wilkinson, T. Mayor, P. Mortensen, E.A. Nigg, and M. Mann. 2003. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 426:570–574. - PubMed
- Avidor-Reiss, T., A.M. Maer, E. Koundakjian, A. Polyanovsky, T. Keil, S. Subramaniam, and C.S. Zuker. 2004. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 117:527–539. - PubMed
- Badano, J.L., N. Mitsuma, P.L. Beales, and N. Katsanis. 2006. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7:125–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials