gammaH2AX foci form preferentially in euchromatin after ionising-radiation - PubMed (original) (raw)
gammaH2AX foci form preferentially in euchromatin after ionising-radiation
Ian G Cowell et al. PLoS One. 2007.
Abstract
Background: The histone variant histone H2A.X comprises up to 25% of the H2A complement in mammalian cells. It is rapidly phosphorylated following exposure of cells to double-strand break (DSB) inducing agents such as ionising radiation. Within minutes of DSB generation, H2AX molecules are phosphorylated in large chromatin domains flanking DNA double-strand breaks (DSBs); these domains can be observed by immunofluorescence microscopy and are termed gammaH2AX foci. H2AX phosphorylation is believed to have a role mounting an efficient cellular response to DNA damage. Theoretical considerations suggest an essentially random chromosomal distribution of X-ray induced DSBs, and experimental evidence does not consistently indicate otherwise. However, we observed an apparently uneven distribution of gammaH2AX foci following X-irradiation with regions of the nucleus devoid of foci.
Methodology/principle findings: Using immunofluorescence microscopy, we show that focal phosphorylation of histone H2AX occurs preferentially in euchromatic regions of the genome following X-irradiation. H2AX phosphorylation has also been demonstrated previously to occur at stalled replication forks induced by UV radiation or exposure to agents such as hydroxyurea. In this study, treatment of S-phase cells with hydroxyurea lead to efficient H2AX phosphorylation in both euchromatin and heterochromatin at times when these chromatin compartments were undergoing replication. This suggests a block to H2AX phosphorylation in heterochromatin that is at least partially relieved by ongoing DNA replication.
Conclusions/significance: We discuss a number of possible mechanisms that could account for the observed pattern of H2AX phosphorylation. Since gammaH2AX is regarded as forming a platform for the recruitment or retention of other DNA repair and signaling molecules, these findings imply that the processing of DSBs in heterochromatin differs from that in euchromatic regions. The differential responses of heterochromatic and euchromatic compartments of the genome to DSBs will have implications for understanding the processes of DNA repair in relation to nuclear and chromatin organization.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
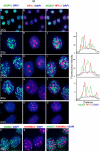
Figure 1. γH2AX foci induced by ionizing radiation are absent from HP1α-staining nuclear domains.
MCF7 cells were fixed 30 minutes after X-irradiation (2Gy, panels a–l & p–r) and were processed for immunofluorescence for γH2AX (green) and either HP1α or H3K9Me3 (red). Panels m–o, non-irradiated cells. DNA was stained with DAPI. Panels a, d, g, j, m & p, γH2AX; panels b, e, h, k & n, HP1α; Panel q, H3K9Me3; panels c, f, I, l, o & r merged images. Overlapping red and green signals appear yellow. The top row shows a group of cells with typical appearance. Individual nuclei are shown magnified in rows 2–6. For the nuclei shown in rows 2–4, line traces were generated (shown on the right) with the line drawn through the brightest HP1α regions. For γH2AX, HP1α and H3K9Me3, images were first adjusted using levels such that fainter interfocal nuclear fluorescence was not included.
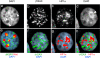
Figure 2. γH2AX foci generated by etoposide treatment do not appear in HP1α-staining regions in MCF7 cells.
MCF7 cells were incubated in medium containing etoposide and processed as in Figure 1. Panels a–c, two hour exposure to 5 µM etoposide. Panels d–i, 24 hour exposure to 1 µM etoposide. Panels j–l, untreated cells. Cells were fixed immediately after etoposide treatment.
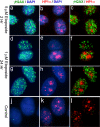
Figure 3. Spatial relationship between γH2AX foci, HP1α-staining heterochromatic regions and nucleoli.
Panel b–d and f–h were obtained from a single nucleus fixed 30 minutes after X-irradiation (2Gy) and processed for γH2AX and HP1α immunofluorescence. Panels, a & e were obtained from a separate irradiated nucleus processed for nucleolin and γH2AX immunofluorescence. Panel a, DAPI; b, γH2AX; c, HP1α; d, DAPI; e, merged DAPI (blue) and nucleolin (red), γH2AX (green) images; f, γH2AX (green)/DAPI (blue); g, HP1α (red)/DAPI (blue); h, γH2AX/HP1α/DAPI. In panels e–h, the red and green chanels were reduced to binary images, retaining as much detail as possible, before overlaying on the DAPI image. DAPI staining was carried out under optimum conditions to reveal nuclear structure.
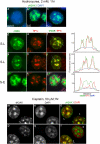
Figure 4. Replication stress can induce phosphorylation of histone H2AX in heterochromatin.
Panels a–l, subconfluent asynchronous MCF7 cells were exposed to hydroxyurea (2 mM) for one hour immediately prior to fixation and processing for γH2AX (green) and HP1α (red) immunofluorescence. Panels a–c, representative nuclei displaying non-S phase, early to mid S-phase (S-E) and late S-phase (S-L) γH2AX staining respectively. Panels d–f & g–I, single S-L nuclei; j–l, single S-E nucleus. Panels d, g, j, γH2AX; e, h, k, HP1α; f, i, l, merged γH2AX/HP1α images. Line traces are presented on the right. Lines were drawn across the nucleus through heterochromatic (HP1α staining) regions in each case, including the DAPI channel. Panels m–r, subconfluent MCF7 cells were exposed to cisplatin (50 µM) for one hour, 38 hours after release from serum starvation. Cells were fixed immediately after cisplatin treatment and processed for γH2AX immunofluorescence. Panels m & p, γH2AX; n & q, DAPI; o & r merged γH2AX (green)/DAPI (red) images.
Figure 5. G1 and S-phase H2AX phosphorylation.
MCF7 cells were serum starved (0.05% FCS) for 24 hours before release into medium containing 20% FCS. Cells were fixed at different times such that fixed cell populations were predominantly in G1, early S (S-E) or late S-phase (S-L). (6.5 hr, 24 hr and 38 hr respectively). HU or cisplatin were added to the cells, to 2 mM or 50 µM respectively, at the indicated times prior to fixation. Irradiated cells were fixed 30 minutes after irradiation.
Similar articles
- DNA double-strand breaks in heterochromatin elicit fast repair protein recruitment, histone H2AX phosphorylation and relocation to euchromatin.
Jakob B, Splinter J, Conrad S, Voss KO, Zink D, Durante M, Löbrich M, Taucher-Scholz G. Jakob B, et al. Nucleic Acids Res. 2011 Aug;39(15):6489-99. doi: 10.1093/nar/gkr230. Epub 2011 Apr 21. Nucleic Acids Res. 2011. PMID: 21511815 Free PMC article. - Evaluation of the spatial distribution of gammaH2AX following ionizing radiation.
Vasireddy RS, Tang MM, Mah LJ, Georgiadis GT, El-Osta A, Karagiannis TC. Vasireddy RS, et al. J Vis Exp. 2010 Aug 7;(42):2203. doi: 10.3791/2203. J Vis Exp. 2010. PMID: 20736911 Free PMC article. - Preferential localization of γH2AX foci in euchromatin of retina rod cells after DNA damage induction.
Lafon-Hughes L, Di Tomaso MV, Liddle P, Toledo A, Reyes-Ábalos AL, Folle GA. Lafon-Hughes L, et al. Chromosome Res. 2013 Dec;21(8):789-803. doi: 10.1007/s10577-013-9395-3. Epub 2013 Dec 10. Chromosome Res. 2013. PMID: 24323064 - Chromatin dynamics and the repair of DNA double strand breaks.
Xu Y, Price BD. Xu Y, et al. Cell Cycle. 2011 Jan 15;10(2):261-7. doi: 10.4161/cc.10.2.14543. Epub 2011 Jan 15. Cell Cycle. 2011. PMID: 21212734 Free PMC article. Review. - Mechanism of elimination of phosphorylated histone H2AX from chromatin after repair of DNA double-strand breaks.
Svetlova MP, Solovjeva LV, Tomilin NV. Svetlova MP, et al. Mutat Res. 2010 Mar 1;685(1-2):54-60. doi: 10.1016/j.mrfmmm.2009.08.001. Epub 2009 Aug 12. Mutat Res. 2010. PMID: 19682466 Review.
Cited by
- Model for MLL translocations in therapy-related leukemia involving topoisomerase IIβ-mediated DNA strand breaks and gene proximity.
Cowell IG, Sondka Z, Smith K, Lee KC, Manville CM, Sidorczuk-Lesthuruge M, Rance HA, Padget K, Jackson GH, Adachi N, Austin CA. Cowell IG, et al. Proc Natl Acad Sci U S A. 2012 Jun 5;109(23):8989-94. doi: 10.1073/pnas.1204406109. Epub 2012 May 21. Proc Natl Acad Sci U S A. 2012. PMID: 22615413 Free PMC article. - Stwl modifies chromatin compaction and is required to maintain DNA integrity in the presence of perturbed DNA replication.
Yi X, de Vries HI, Siudeja K, Rana A, Lemstra W, Brunsting JF, Kok RM, Smulders YM, Schaefer M, Dijk F, Shang Y, Eggen BJ, Kampinga HH, Sibon OC. Yi X, et al. Mol Biol Cell. 2009 Feb;20(3):983-94. doi: 10.1091/mbc.e08-06-0639. Epub 2008 Dec 3. Mol Biol Cell. 2009. PMID: 19056684 Free PMC article. - Depletion of Histone Demethylase Jarid1A Resulting in Histone Hyperacetylation and Radiation Sensitivity Does Not Affect DNA Double-Strand Break Repair.
Penterling C, Drexler GA, Böhland C, Stamp R, Wilke C, Braselmann H, Caldwell RB, Reindl J, Girst S, Greubel C, Siebenwirth C, Mansour WY, Borgmann K, Dollinger G, Unger K, Friedl AA. Penterling C, et al. PLoS One. 2016 Jun 2;11(6):e0156599. doi: 10.1371/journal.pone.0156599. eCollection 2016. PLoS One. 2016. PMID: 27253695 Free PMC article. - New paradigms in the repair of oxidative damage in human genome: mechanisms ensuring repair of mutagenic base lesions during replication and involvement of accessory proteins.
Dutta A, Yang C, Sengupta S, Mitra S, Hegde ML. Dutta A, et al. Cell Mol Life Sci. 2015 May;72(9):1679-98. doi: 10.1007/s00018-014-1820-z. Epub 2015 Jan 10. Cell Mol Life Sci. 2015. PMID: 25575562 Free PMC article. Review. - Chromatin dynamics in DNA double-strand break repair.
Shi L, Oberdoerffer P. Shi L, et al. Biochim Biophys Acta. 2012 Jul;1819(7):811-9. doi: 10.1016/j.bbagrm.2012.01.002. Epub 2012 Jan 17. Biochim Biophys Acta. 2012. PMID: 22285574 Free PMC article. Review.
References
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. - PubMed
- Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, et al. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. - PubMed
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. - PubMed
- Shiloh Y. The ATM-mediated DNA-damage response: taking shape. Trends Biochem Sci. 2006;31:402–410. - PubMed
- Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, et al. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources