Loss of Bardet Biedl syndrome proteins causes defects in peripheral sensory innervation and function - PubMed (original) (raw)
. 2007 Oct 30;104(44):17524-9.
doi: 10.1073/pnas.0706618104. Epub 2007 Oct 24.
Travis Barr, Peter N Inglis, Norimasa Mitsuma, Susan M Huang, Miguel A Garcia-Gonzalez, Brian A Bradley, Stephanie Coforio, Phillip J Albrecht, Terry Watnick, Gregory G Germino, Philip L Beales, Michael J Caterina, Michel R Leroux, Frank L Rice, Nicholas Katsanis
Affiliations
- PMID: 17959775
- PMCID: PMC2077289
- DOI: 10.1073/pnas.0706618104
Loss of Bardet Biedl syndrome proteins causes defects in peripheral sensory innervation and function
Perciliz L Tan et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Reception and interpretation of environmental stimuli is critical for the survival of all organisms. Here, we show that the ablation of BBS1 and BBS4, two genes mutated in Bardet-Biedl syndrome and that encode proteins that localize near the centrioles of sensory neurons, leads to alterations of s.c. sensory innervation and trafficking of the thermosensory channel TRPV1 and the mechanosensory channel STOML3, with concomitant defects in peripheral thermosensation and mechanosensation. The thermosensory phenotype is recapitulated in Caenorhabditis elegans, because BBS mutants manifest deficient thermosensory responses at both physiological and nociceptive temperatures and defective trafficking of OSM-9, a polymodal sensory channel protein and a functional homolog of TRPV1 or TRPV4. Our findings suggest a hitherto unrecognized, but essential, role for mammalian basal body proteins in the acquisition of mechano- and thermosensory stimuli and highlight potentially clinical features of ciliopathies in humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
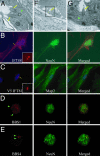
DRG neurons have primary cilia. (A, F, and G) Murine DRGs corresponding to lumbar regions L3–L5 were dissected and subjected to electron microscopy; magnification, ×60,000 (Left); ×8,000 (Center); ×15,000 (Right). (B and C) Dissociated murine DRGs, were grown in culture for 3–7 days, followed by immunocytochemistry using the intraflagellar transport marker Polaris (IFT88) (B) along with a neuronal marker NeuN. A 72-h transfection of IFT81 tagged with V5 was also stained with an anti-V5 antibody and MAP2 (C), a dendritic marker. Yellow arrows point to cilia. (Inset) Zoom view of the neuronal cilium staining. (D and E) Immunocytochemistry of cultured DRG neurons with antibodies against BBS1 (D) and BBS4 (E), costained with the neuronal marker NeuN. Green arrowheads point out positive basal body localization.
Fig. 2.
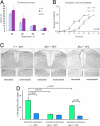
_Bbs1_−/− mice have deficient responses to thermal and mechanical stimuli as well as reduced signal transduction to the spinal cord in response to thermal stimulus. (A) Results from the tail immersion assay in which continuous stimulus at 46°C, 48°C, 50°C, or 52°C is applied, and the time to respond is recorded. Overall, _Bbs1_−/− animals had greater latencies than their WT counterparts. (B) The von Frey assay on _Bbs1_−/− mice show a greater amount of force needed to induce a mechanical response compared with WT. (C) Representative sections of the L3/L4/L5 dorsal horn region of Bbs1+/+ and _Bbs1_−/− mice stained with anti-c-FOS after stimulation of one hind paw in a 48°C or 52°C water bath. (D) Plot of the number of _c-Fos_-positive nuclei in the ipsilateral dorsal horn versus the contralateral dorsal horn after stimulation of one hind paw in a 48°C or 52°C water bath (n = 4 mice per genotype). (Magnification, ×10.)
Fig. 3.
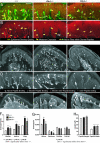
Differences in epidermal structure and innervation in _Bbs_−/− mice. (A and B) Images of distal pads from glabrous hind paws showing CGRP (red) with PGP (green) (A) and CGRP (B) immunolabeling alone. _Bbs_−/− animals had reduced CGRP immunoreactivity in nerve fibers within dermal papillae (yellow arrows), whereas no differences were evident in the proportion of CGRP-positive epidermal endings (yellow stars). Green arrows and stars indicate PGP-positive dermal nerve fibers and epidermal endings, respectively. The epidermal–dermal border is indicated by a broken line. (C) Images of distal pads from WT and _Bbs_−/− mice labeled for PGP 9.5. Innervation is evenly spaced throughout the epidermis of the WT pad but is concentrated at dermal papillae in Bbs mutants. (D) Close-up of the selected distal pad region illustrating the subjective classification of sensory endings based on their position in the epidermis relative to dermal papillae (between, above, or at the border of a papilla). Endings spanning multiple regions were labeled for each region they passed through (e.g., border interpapilla endings). (E) Images of distal pads labeled with anti-mouse Cy2 to delineate the dermal–epidermal border showing that Bbs mutants have large, misshaped dermal papillae (white arrowheads). Papillae (solid white lines) and examples of the subjectively determined regions above (dashed white lines) and at the border of a papilla (dotted white lines) are traced. (F–H) Quantification of epidermal innervation and dermal papilla structure conducted on tissue sections labeled with PGP 9.5 and mouse anti-Cy2. (F) Both _Bbs_−/− mutants had significantly more endings above and at the border of papillae compared with WT, and _Bbs1_−/− mice had significantly more total papillae endings than _Bbs4_−/− mice. Total papillae endings were the sum of all endings that passed through the regions above or at the border of papillae. (G) _Bbs1_−/− mice had a significantly greater density of endings above their papillae, and _Bbs4_−/− mice had significantly fewer endings between their papillae, indicating that the higher number of papillae-associated endings in these animals was not merely due to changes in papillae size. No significant differences were found for ending densities outside the papillae region (from beginning and end of the pad to the first papilla) or over the entire pad. Epidermal ending densities were calculated by dividing the number of endings in a region by its length. (H) Both Bbs mutants had significantly longer papillae widths but not heights. Papilla height was measured from its estimated base to its peak height in the epidermis, and width was calculated by dividing the area of the papilla by its height. dp, dermal papilla, sg, sweat gland; e, epidermis; d, dermis.
Fig. 4.
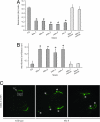
C. elegans ciliary mutants show thermotaxis and thermal avoidance defects as well as mislocalized OSM-9. (A) Thermotaxis and thermal avoidance of WT (N2), bbs mutants, and osm-3 kinesin mutants. Thermotaxis relates to how well the worms move to their rearing temperature zone (20°C). (B) Nonthermal avoidance is the proportion of worms that do not avoid a noxious temperature presented near the head of the animal. For A and B, *, P < 0.00001. (C) GFP-tagged OSM-9 mislocalizes in bbs-8 mutant animals. TZ, transition zone/basal body; *, abnormal accumulations not seen in wild-type animals.
Fig. 5.
Distribution of STOML3 in DRG neurons. In contrast to WT animals, STOML3 staining is perturbed in BBS mutants, where it appears to be mistrafficked to the nucleus. Images are at magnification, ×40.
Similar articles
- Mutations in a guanylate cyclase GCY-35/GCY-36 modify Bardet-Biedl syndrome-associated phenotypes in Caenorhabditis elegans.
Mok CA, Healey MP, Shekhar T, Leroux MR, Héon E, Zhen M. Mok CA, et al. PLoS Genet. 2011 Oct;7(10):e1002335. doi: 10.1371/journal.pgen.1002335. Epub 2011 Oct 13. PLoS Genet. 2011. PMID: 22022287 Free PMC article. - Hyperactive neuroendocrine secretion causes size, feeding, and metabolic defects of C. elegans Bardet-Biedl syndrome mutants.
Lee BH, Liu J, Wong D, Srinivasan S, Ashrafi K. Lee BH, et al. PLoS Biol. 2011 Dec;9(12):e1001219. doi: 10.1371/journal.pbio.1001219. Epub 2011 Dec 13. PLoS Biol. 2011. PMID: 22180729 Free PMC article. - Gene Therapeutic Reversal of Peripheral Olfactory Impairment in Bardet-Biedl Syndrome.
Williams CL, Uytingco CR, Green WW, McIntyre JC, Ukhanov K, Zimmerman AD, Shively DT, Zhang L, Nishimura DY, Sheffield VC, Martens JR. Williams CL, et al. Mol Ther. 2017 Apr 5;25(4):904-916. doi: 10.1016/j.ymthe.2017.02.006. Epub 2017 Feb 22. Mol Ther. 2017. PMID: 28237838 Free PMC article. - Bardet-Biedl syndrome: Is it only cilia dysfunction?
Novas R, Cardenas-Rodriguez M, Irigoín F, Badano JL. Novas R, et al. FEBS Lett. 2015 Nov 14;589(22):3479-91. doi: 10.1016/j.febslet.2015.07.031. Epub 2015 Jul 29. FEBS Lett. 2015. PMID: 26231314 Review. - [Current status and implication of research on Bardet-Biedl syndrome].
Shen T, Yan XM, Xiao CJ. Shen T, et al. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2013 Oct;30(5):570-3. doi: 10.3760/cma.j.issn.1003-9406.2013.05.013. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2013. PMID: 24078572 Review. Chinese.
Cited by
- The Bardet-Biedl syndrome-related protein CCDC28B modulates mTORC2 function and interacts with SIN1 to control cilia length independently of the mTOR complex.
Cardenas-Rodriguez M, Irigoín F, Osborn DP, Gascue C, Katsanis N, Beales PL, Badano JL. Cardenas-Rodriguez M, et al. Hum Mol Genet. 2013 Oct 15;22(20):4031-42. doi: 10.1093/hmg/ddt253. Epub 2013 May 31. Hum Mol Genet. 2013. PMID: 23727834 Free PMC article. - The primary cilium as a complex signaling center.
Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. Berbari NF, et al. Curr Biol. 2009 Jul 14;19(13):R526-35. doi: 10.1016/j.cub.2009.05.025. Curr Biol. 2009. PMID: 19602418 Free PMC article. Review. - Temperature-dependent behaviors of parasitic helminths.
Bryant AS, Hallem EA. Bryant AS, et al. Neurosci Lett. 2018 Nov 20;687:290-303. doi: 10.1016/j.neulet.2018.10.023. Epub 2018 Oct 15. Neurosci Lett. 2018. PMID: 30336196 Free PMC article. Review. - A Glial K/Cl Transporter Controls Neuronal Receptive Ending Shape by Chloride Inhibition of an rGC.
Singhvi A, Liu B, Friedman CJ, Fong J, Lu Y, Huang XY, Shaham S. Singhvi A, et al. Cell. 2016 May 5;165(4):936-48. doi: 10.1016/j.cell.2016.03.026. Epub 2016 Apr 7. Cell. 2016. PMID: 27062922 Free PMC article. - cGMP dynamics that underlies thermosensation in temperature-sensing neuron regulates thermotaxis behavior in C. elegans.
Aoki I, Shiota M, Tsukada Y, Nakano S, Mori I. Aoki I, et al. PLoS One. 2022 Dec 6;17(12):e0278343. doi: 10.1371/journal.pone.0278343. eCollection 2022. PLoS One. 2022. PMID: 36472979 Free PMC article.
References
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zucer CS. Cell. 2004;117:527–539. - PubMed
- Blacque OE, Perens EA, Boroevich KA, Inglis PN, Li C, Warner A, Khattra J, Holt RA, Ou G, Mah AK, et al. Curr Biol. 2005;15:935–941. - PubMed
- Mori I. Annu Rev Genet. 1999;33:399–422. - PubMed
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Dev Biol. 1986;117:456–487. - PubMed
- Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, Lupski JR, Beales PL, Reed RR, Katsanis N. Nat Genet. 2004;36:994–998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources