NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons - PubMed (original) (raw)
Comparative Study
NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons
Houman Homayoun et al. J Neurosci. 2007.
Abstract
NMDA receptors mediate excitatory postsynaptic potentials throughout the brain but, paradoxically, NMDA receptor antagonists produce cortical excitation in humans and behaving rodents. To elucidate a mechanism for these diverging effects, we examined the effect of use-dependent inhibition of NMDA receptors on the spontaneous activity of putative GABA interneurons and pyramidal neurons in the prefrontal cortex of awake rats. We find that inhibition of NMDA receptors predominately decreases the activity of putative GABA interneurons but, at a delayed rate, increases the firing rate of the majority of pyramidal neurons. Thus, NMDA receptors preferentially drive the activity of cortical inhibitory interneurons suggesting that NMDA receptor inhibition causes cortical excitation by disinhibition of pyramidal neurons. These findings support the hypothesis that NMDA receptor hypofunction, which has been implicated in the pathophysiology of schizophrenia, diminishes the inhibitory control of PFC output neurons. Reducing this effect may be critical for treatment of schizophrenia.
Figures
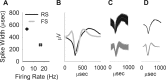
Figure 1.
Distinction between putative interneurons (FS units) and pyramidal neurons (RS units). A, The population of putative interneurons had a higher firing rate (abscissa) and narrower spikeform (ordinate, average ± SEM of peak to valley spike width). B, Average waveforms of representative RS and FS units. C, Superimposed composite of all the waveforms for the same units. D, Superimposed average waveforms during two 10 min windows, pre- and post-MK801 administration. The waveforms remained stable after MK801 treatment compared with the predrug period.
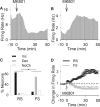
Figure 2.
Inhibitory effects of MK801 on putative interneurons. A, B, Examples of inhibitory and excitatory effects of MK801 on an individual interneuron (A) and a pyramidal neuron (B). Each panel depicts the firing rate histogram (bin, 1 min; smoothed with a Gaussian filter with bin width of 3) of a single unit. Arrow indicates the time of MK801 injection. C, Distribution of firing rate responses (increase, decrease, or no change) to MK801 was significantly different among populations of RS and FS units (*p < 0.001). D, Comparison of average population response of RS and FS units to MK801. Two-way ANOVA with time as the repeated measure revealed a significant effect for neuron type (F(1,259) = 12.96; p < 0.001), time (F(59,15281) = 2.71; p < 0.001), and type by time interaction (F(59,15281) = 5.94; p < 0.001). The averages for all FS units and those with a decreased response type have been depicted as dashed and solid gray lines, respectively.
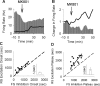
Figure 3.
MK801 inhibition of interneurons precedes its excitation of pyramidal cells. A, Superimposed firing-rate histogram of simultaneously recorded pairs of interneurons (bar graph) and pyramidal neurons (solid line). Arrow indicates the time of MK801 injection. Bin size, 1 min. B, Normalized firing rate histogram of another pair of simultaneously recorded interneuron (bar graph; baseline firing rate, 17.26 Hz) and pyramidal neuron (solid line; baseline, 2.86 Hz). Inset, Finer timescale with 20 s bins. C, D, Putative interneurons preceded their concurrently recorded pyramidal neurons both in the onset of their inhibition (C) and in reaching the inhibitory plateau (D). Each dot in the scatter plots depicts the post-MK801 delay to onset (C) or plateau (D) of inhibitory response for interneuron/s recorded during a single session versus the delay to onset (C) or plateau (D) of excitatory response for the average pyramidal neurons in the same session. A dashed line indicates no change from baseline and a solid line depicts the linear regression. Inset, Median and 95% confidence intervals of the difference in the delay between inhibitory versus excitatory response onset (C) or plateau (D), with a positive value indicating inhibition preceding the excitation (_y_-axis range, 0–1500 s).
Similar articles
- Prolonged exposure to NMDAR antagonist induces cell-type specific changes of glutamatergic receptors in rat prefrontal cortex.
Wang HX, Gao WJ. Wang HX, et al. Neuropharmacology. 2012 Mar;62(4):1808-22. doi: 10.1016/j.neuropharm.2011.11.024. Epub 2011 Dec 9. Neuropharmacology. 2012. PMID: 22182778 Free PMC article. - NMDA receptor antagonists disinhibit rat posterior cingulate and retrosplenial cortices: a potential mechanism of neurotoxicity.
Li Q, Clark S, Lewis DV, Wilson WA. Li Q, et al. J Neurosci. 2002 Apr 15;22(8):3070-80. doi: 10.1523/JNEUROSCI.22-08-03070.2002. J Neurosci. 2002. PMID: 11943810 Free PMC article. - NMDA receptor activation enhances inhibitory GABAergic transmission onto hippocampal pyramidal neurons via presynaptic and postsynaptic mechanisms.
Xue JG, Masuoka T, Gong XD, Chen KS, Yanagawa Y, Law SK, Konishi S. Xue JG, et al. J Neurophysiol. 2011 Jun;105(6):2897-906. doi: 10.1152/jn.00287.2010. Epub 2011 Apr 6. J Neurophysiol. 2011. PMID: 21471392 - NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia.
Gonzalez-Burgos G, Lewis DA. Gonzalez-Burgos G, et al. Schizophr Bull. 2012 Sep;38(5):950-7. doi: 10.1093/schbul/sbs010. Epub 2012 Feb 21. Schizophr Bull. 2012. PMID: 22355184 Free PMC article. Review. - Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia.
Lewis DA, Curley AA, Glausier JR, Volk DW. Lewis DA, et al. Trends Neurosci. 2012 Jan;35(1):57-67. doi: 10.1016/j.tins.2011.10.004. Epub 2011 Dec 6. Trends Neurosci. 2012. PMID: 22154068 Free PMC article. Review.
Cited by
- Electrophysiological intermediate biomarkers for oxidative stress in schizophrenia.
Ballesteros A, Summerfelt A, Du X, Jiang P, Chiappelli J, Tagamets M, O'Donnell P, Kochunov P, Hong LE. Ballesteros A, et al. Clin Neurophysiol. 2013 Nov;124(11):2209-15. doi: 10.1016/j.clinph.2013.05.021. Epub 2013 Jun 30. Clin Neurophysiol. 2013. PMID: 23823132 Free PMC article. Clinical Trial. - Sex-specific effects of subchronic NMDA receptor antagonist MK-801 treatment on hippocampal gamma oscillations.
Neuhäusel TS, Gerevich Z. Neuhäusel TS, et al. Front Neurosci. 2024 Aug 7;18:1425323. doi: 10.3389/fnins.2024.1425323. eCollection 2024. Front Neurosci. 2024. PMID: 39170673 Free PMC article. - Effects of Ketamine on Resting-State EEG Activity and Their Relationship to Perceptual/Dissociative Symptoms in Healthy Humans.
de la Salle S, Choueiry J, Shah D, Bowers H, McIntosh J, Ilivitsky V, Knott V. de la Salle S, et al. Front Pharmacol. 2016 Sep 27;7:348. doi: 10.3389/fphar.2016.00348. eCollection 2016. Front Pharmacol. 2016. PMID: 27729865 Free PMC article. - Tonic NMDA receptor-mediated current in prefrontal cortical pyramidal cells and fast-spiking interneurons.
Povysheva NV, Johnson JW. Povysheva NV, et al. J Neurophysiol. 2012 Apr;107(8):2232-43. doi: 10.1152/jn.01017.2011. Epub 2012 Jan 11. J Neurophysiol. 2012. PMID: 22236713 Free PMC article. - NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex.
Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF. Wang M, et al. Neuron. 2013 Feb 20;77(4):736-49. doi: 10.1016/j.neuron.2012.12.032. Neuron. 2013. PMID: 23439125 Free PMC article.
References
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156:1646–1649. - PubMed
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. - PubMed
- Arvanov VL, Wang RY. NMDA-induced response in pyramidal neurons of the rat medial prefrontal cortex slices consists of NMDA and non-NMDA components. Brain Res. 1997;768:361–364. - PubMed
- Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiat. 1997;154:805–811. - PubMed
- Chen L, Muhlhauser M, Yang CR. Glycine tranporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol. 2003;89:691–703. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous