Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation - PubMed (original) (raw)
Comparative Study
Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation
Lenora J Volk et al. J Neurosci. 2007.
Abstract
Gq-coupled, M1 muscarinic acetylcholine receptors (mAChRs) facilitate hippocampal learning, memory, and synaptic plasticity. M1 mAChRs induce long-term synaptic depression (LTD), but little is known about the underlying mechanisms of mAChR-dependent LTD and its link to cognitive function. Here, we demonstrate that chemical activation of M1 mAChRs induces LTD in hippocampal area CA1, which relies on rapid protein synthesis, as well as the extracellular signal-regulated kinase and mammalian target of rapamycin translational activation pathways. Synaptic stimulation of M1 mAChRs, alone, or together with the Gq-coupled glutamate receptors (mGluRs), also results in protein synthesis-dependent LTD. New proteins maintain mAChR-dependent LTD through a persistent decrease in surface AMPA receptors. mAChRs stimulate translation of the RNA-binding protein, Fragile X mental retardation protein (FMRP) and FMRP target mRNAs. In mice without FMRP (Fmr1 knock-out), a model for human Fragile X syndrome mental retardation (FXS), both mGluR- and mAChR-dependent protein synthesis and LTD are affected. Our results reveal that multiple Gq-coupled receptors converge on a common protein synthesis-dependent LTD mechanism, which is aberrant in FXS. These findings suggest novel therapeutic strategies for FXS in the form of mAChR antagonists.
Figures
Figure 1.
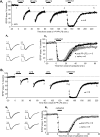
Muscarinic acetylcholine receptor-induced long-term synaptic depression requires protein synthesis, ERK, and mTOR activity. A–E, Application of the muscarinic acetylcholine receptor agonist CCh (50 μ
m
, 10 min) to acute hippocampal slices from mature rats (P25–P41) induces a long-term depression of EPSCs (A) and fEPSPs (B–E). Plotted are average (±SEM) amplitude values of EPSCs or average initial slope values of fEPSPs normalized to the pre-CCh baseline. All drugs except CCh were present in the bath throughout the experiment. All experiments are compared with interleaved controls. A, Stimulation of mAChRs induces LTD (EPSC amplitude at 55–60 = 71 ± 4% of baseline; p = 0.002), which does not require cell depolarization (cells were clamped at −60 mV for the duration of the experiment). Calibration: 100 pA, 10 ms. B, The protein synthesis inhibitor anisomycin (25 μ
m
) significantly reduces CCh-induced LTD (control, 70 ± 2%; anisomycin, 87 ± 3%; p < 0.001). LTD is quantified as a 5 min average taken 1 h after CCh washout, and all statistical comparisons are made at this time point (see Materials and Methods). Inset, fEPSP waveforms (average of 4–6 traces) from a representative experiment are taken at the time points indicated on the graph (1, 2). Calibration: 0.5 mV, 5 ms. C, The transcription inhibitor actinomycin D (25 μ
m
) has no effect on CCh-LTD [control (0.1% DMSO), 81 ± 4%; actinomycin D, 80 ± 3%; p = 0.8]. D, The mTOR antagonist rapamycin (20 n
m
) reduces CCh-induced LTD [control (0.1% DMSO), 69 ± 5%; rapamycin, 87 ± 3%; p < 0.01). E, The MEK inhibitor U0126 (20 μ
m
) blocks CCh-induced LTD [control (0.1% DMSO), 82 ± 2%; U0126, 99 ± 3; control vs U1026, p < 0.001; U0126 1 h after CCh washout vs U0126 baseline, p = 0.7]. F, Acute rat hippocampal slices (CA3 removed) were treated ±10 min with 50 μ
m
CCh. After treatment, CA1 was microdissected on ice, and samples were immediately frozen on dry ice and processed for Western blotting. Right panel, Representative Western blots. P-S6K, phospho-p70 S6 kinase; P-ERK, phospho ERK; T-ERK, total ERK. Middle panel, Quantification of CCh-induced activation of p70 S6 kinase at the rapamycin-sensitive site (Thr389) (P-S6K/T-ERK, % control) in nine slices (control, 100 ± 2%; CCh, 171 ± 6%; **p < 0.001). Left panel, Quantification of CCh-induced ERK activation (P-ERK/T-ERK, % control) in nine slices (control, 100 ± 5%; CCh, 293 ± 23%; **p < 0.001).
Figure 2.
Protein synthesis-dependent CCh-induced LTD does not require activity of NMDARs, group 1 mGluRs, or presynaptic stimulation. A, Preincubation of slices in the NMDA receptor antagonist,
d
,
l
-AP-5 (100 μ
m
) before CCh had no effect on LTD (control, 82 ± 5%; AP-5, 77 ± 2%; p = 0.3). AP-5 was effective in blocking LTP induction with a theta-burst stimulation (θ; ↑) delivered after CCh-LTD induction (measured 25 min after theta burst; control, 129 ± 7%; AP-5 = 99 ± 1%; θ in AP-5, p = 0.77 vs baseline; θ in control, p = 0.01 vs baseline). fEPSP slope values are renormalized to pre-theta baseline. B, Cessation of presynaptic stimulation immediately before and for 30 min after CCh application (CCh only) had no effect on LTD magnitude compared with slices in the same recording chamber, which received continuous stimulation during CCh (CCh + stim) (CCh + stim, 83 ± 2%; CCh no stim, 80 ± 3%; p = 0.4.). C, Blockade of mGluR5 and mGluR1 with MPEP (10 μ
m
) and LY367385 (100 μ
m
), respectively, had no effect on LTD induced with CCh (control, 87 ± 1; MPEP + LY, 81 ± 4%; p = 0.2).
Figure 3.
Synaptically induced protein synthesis-dependent LTD requires M1 mAChR activation. All experiments are in the presence of the NMDA receptor antagonist
d
,
l
-AP-5 (100 μ
m
). A, Blockade of mAChRs with the nonselective muscarinic antagonist atropine (5 μ
m
) reduces LTD induced synaptically with paired-pulse low-frequency stimulation (PP-LFS, 2 pulses with 50 ms interstimulus interval delivered at 1 Hz; 20 min) (measured 1 h after PP-LFS, control, 78 ± 3%; atropine, 90 ± 2%; p = 0.01). B, Blockade of the M1 subtype of mAChRs with the M1-selective antagonist pirenzepine (75 n
m
) reduces PP-LFS induced LTD (measured 1 h after PP-LFS; control, 75 ± 3%; pirenzepine, 88 ± 4%; p < 0.01). C, Brief application of the acetylcholine esterase inhibitor eserine (2 μ
m
; 5 min before and during PP-LFS) facilitated LTD induced with PP-LFS (15 min) (measured 70 min after PP-LFS; control, 88 ± 2%; eserine, 78 ± 3%; p = 0.02). D, PP-LFS-induced LTD is blocked by combined antagonism of M1 mAChRs (75 n
m
pirenzepine) and group I mGluRs (mGluR5-specific antagonist MPEP at 10 μ
m
plus mGluR1-specific antagonist, LY367385 at 100 μ
m
), “P,M,L” (measured 1 h after PP-LFS control, 72 ± 4%; “P,M,L”, 96 ± 4%; control vs “P,M,L”, p < 0.001; “P,M,L” at 85–90 min vs baseline, p = 0.37).
Figure 4.
Saturation of synaptically induced, Gq-dependent LTD occludes CCh-induced LTD. A1, Three episodes of PP-LFS (15 min) delivered to Schaffer collateral axons in the presence of
d
,
l
-AP-5 (100 μ
m
) saturated LTD (LTD after second, 70 ± 4%, and third PP-LFS, 69 ± 4%, episode were not different; p = 0.86). LTD induced by CCh after saturation of PP-LFS-induced LTD is dramatically reduced. A2, fEPSP waveforms (average of 4–6 traces) from a representative experiment are taken at the time points indicated on the graph in A1. A3, Previous saturation of PP-LFS-induced LTD reduced subsequent CCh-induced LTD compared with interleaved naive control slices, which received only baseline stimulation (“post PP-LFS” replotted from 130–230 min of A1; measured 1 h after CCh, naive, 77 ± 2%; CCh LTD after PP-LFS saturation, 93 ± 2%; p < 0.001). B1, Three episodes of LFS (15 min) delivered to Schaffer collateral axons saturated LTD (LTD after second, 84 ± 2%, and third PP-LFS, 81 ± 3%, episodes were not different; p = 0.1). Stimulation of mAChRs with CCh-induced normal levels of LTD after LFS saturation. B2, fEPSP waveforms (average of 4–6 traces) from a representative experiment are taken at the time points indicated on the graph in B1. B3, Previous saturation of LFS-induced LTD has no effect on subsequent CCh-induced LTD compared with interleaved naive control slices, which received only baseline stimulation (“post LFS” replotted from 130–230 min of B1; naive, 87 ± 1%; CCh LTD after LFS saturation, 86 ± 1%; p = 0.1)
Figure 5.
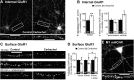
Activation of M1 muscarinic acetylcholine receptors induces endocytosis and long-term, protein synthesis-dependent decreases of surface GluR1. A, Representative experiment in which internalized GluR1 was labeled on live dissociated cultured hippocampal neurons using N-terminal GluR1 antibody (see Materials and Methods). CCh (50 μ
m
; 10 min) treatment increases internalized GluR1. Scale bar, 15 and 10 μm. B, CCh increases the number of internalized GluR1 puncta per 50 μm of proximal dendrite measured 15 min after CCh washout (left panel; control, 100 ± 8%; CCh, 137 ± 8%), which is blocked by the M1 mAChR selective antagonist pirenzepine (75 n
m
) (right panel; control, 100 ± 10%; CCh, 143 ± 13%; pirenzepine, 100 ± 10%; pirenzepine plus CCh, 83 ± 10%; p = 0.2; pirenzepine vs pirenzepine + CCh). Pirenzepine had no effect on basal surface AMPAR expression (p = 0.4). The intensity and area of internalized receptor puncta were unchanged. n (number of cells) is indicated on each bar. Data from two to four cultures per condition. C, Representative experiment in which surface GluR1 was labeled on fixed neurons 10 or 60 min after CCh (50 μ
m
; 10 min) or media (control) treatment (± anisomycin; 25 μ
m
). Scale bar, 10 μm. D, Quantification of surface GluR1 puncta reveals that CCh induces decreases in surface GluR1 at 10 and 60 min after CCh application (10 min: control, 100 ± 6%; CCh, 74 ± 7%; 60 min: control, 100 ± 5%; CCh, 77 ± 6%). Anisomycin blocks the surface GluR1 decreases at 60 min (anisomycin, 100 ± 9%; anisomycin plus CCh, 121 ± 11%; p = 0.2). n (number of cells) indicated on each bar. Data from two to four cultures per condition. *p < 0.05; **p < 0.01. E, M1 mAChRs are expressed and punctate on cultured hippocampal pyramidal neurons. Scale bar, 15 and 10 μm.
Figure 6.
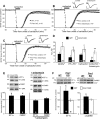
Muscarinic acetylcholine receptor-stimulated LTD and protein synthesis are altered in Fmr1 KO mice. A, CCh (50 μ
m
; 10 min) induced LTD is larger in magnitude in Fmr1 KO mice compared with interleaved experiments in WT mice (WT, 89 ± 2%; Fmr1 KO, 81 ± 3%; p = 0.01). B, As in rats, CCh-LTD is blocked by anisomycin in WT mice (WT control, 88 ± 2%; WT + anisomycin, 97 ± 2%; p < 0.01). C, In contrast, CCh-LTD persists in anisomycin in Fmr1 KO mice (Fmr1 KO control, 85 ± 3%; Fmr1 KO plus anisomycin, 85 ± 2%; p = 0.1). D, Comparison and summary of LTD induced by chemical stimulation of group I mGluRs (DHPG) or muscarinic acetylcholine receptors (carbachol) or synaptic stimulation (PP-LFS) in WT and Fmr1 KO mice. n (number of slices) is indicated on each bar. Data are replotted from the study by Huber et al. (2002). E, Top, Representative Western blots demonstrating that CCh treatment (50 μ
m
; 10 min) induces a rapid and anisomycin-sensitive increase in protein levels for EF1α and FMRP in acute hippocampal slices from WT mice. Total ERK (T-ERK) levels were used as a loading control and were not changed in response to CCh. Bottom, Quantified group data (percentage control) of Western blots (EF1α, 131 ± 10%; FMRP, 128 ± 4%; EF1α + aniso, 93 ± 8%; FMRP + aniso, 109 ± 9%). n (number of mice) is indicated on each bar. *p < 0.05, one-sample t test, percentage control. F, Top, Representative Western blots demonstrating that CCh treatment (50 μ
m
; 10 min) induces an increase in EF1α and αCaMKII protein levels in acute hippocampal slices (isolated CA1) from WT but not Fmr1 KO littermates. VCP levels were used as a loading control and were not changed in response to CCh. Bottom left, Quantified group data (percentage control) of Western blots for EF1α (WT: 167 ± 24%; *p < 0.05; KO, 98 ± 11%; p = 0.5; WT vs KO: p < 0.05). n (number of slices) is indicated on each bar. Bottom right, Quantified group data (percentage control) of Western blots for αCaMKII (WT: 123 ± 6%, *p < 0.05; KO: 101 ± 6%, p = 0.9; WT vs KO: p < 0.05).
Figure 7.
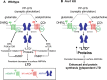
Working model of Gq-dependent LTD in wild-type rodents and Fmr1 KO mice. A, In wild-type rodents, paired pulses of low frequency electrical stimulation (PP-LFS) to Schaffer collateral axons activates both group 1 mGluRs (mGluR1 and mGluR5) and mAChRs to induce LTD. Pharmacological stimulation of group 1 mGluRs (with DHPG) or M1 mAChRs (with CCh) activates each of these pathways individually. Activation of either group 1 mGluRs or mAChRs induces endocytosis of AMPARs and stimulates mRNA translation initiation through the ERK and mTOR pathways (Huber et al., 2000; Banko et al., 2006). Synthesis of proteins, which maintain a reduction in surface AMPARs and LTD are called LTD proteins. mGluRs and mAChRs also stimulate the synthesis of FMRP, which, based on its known function as a translational suppressor, may feedback to inhibit synthesis of LTD proteins. B, In Fmr1 KO mice, mGluRs and mAChRs stimulate endocytosis of AMPARs to induce LTD (Nosyreva and Huber, 2006). In the absence of FMRP, there is a loss of translational suppression and increased steady state level of LTD proteins, which leads to enhanced LTD and AMPAR surface decreases that does not require protein synthesis.
Similar articles
- Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome.
Nosyreva ED, Huber KM. Nosyreva ED, et al. J Neurophysiol. 2006 May;95(5):3291-5. doi: 10.1152/jn.01316.2005. Epub 2006 Feb 1. J Neurophysiol. 2006. PMID: 16452252 - Impaired activity-dependent FMRP translation and enhanced mGluR-dependent LTD in Fragile X premutation mice.
Iliff AJ, Renoux AJ, Krans A, Usdin K, Sutton MA, Todd PK. Iliff AJ, et al. Hum Mol Genet. 2013 Mar 15;22(6):1180-92. doi: 10.1093/hmg/dds525. Epub 2012 Dec 18. Hum Mol Genet. 2013. PMID: 23250915 Free PMC article. - Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered Arc translation and long-term depression.
Niere F, Wilkerson JR, Huber KM. Niere F, et al. J Neurosci. 2012 Apr 25;32(17):5924-36. doi: 10.1523/JNEUROSCI.4650-11.2012. J Neurosci. 2012. PMID: 22539853 Free PMC article. - From FMRP function to potential therapies for fragile X syndrome.
Sethna F, Moon C, Wang H. Sethna F, et al. Neurochem Res. 2014 Jun;39(6):1016-31. doi: 10.1007/s11064-013-1229-3. Epub 2013 Dec 18. Neurochem Res. 2014. PMID: 24346713 Free PMC article. Review. - The translation of translational control by FMRP: therapeutic targets for FXS.
Darnell JC, Klann E. Darnell JC, et al. Nat Neurosci. 2013 Nov;16(11):1530-6. doi: 10.1038/nn.3379. Epub 2013 Apr 14. Nat Neurosci. 2013. PMID: 23584741 Free PMC article. Review.
Cited by
- Phosphorylation of FMRP and alterations of FMRP complex underlie enhanced mLTD in adult rats triggered by early life seizures.
Bernard PB, Castano AM, O'Leary H, Simpson K, Browning MD, Benke TA. Bernard PB, et al. Neurobiol Dis. 2013 Nov;59:1-17. doi: 10.1016/j.nbd.2013.06.013. Epub 2013 Jul 2. Neurobiol Dis. 2013. PMID: 23831253 Free PMC article. - Loss of synaptopodin impairs mGluR5 and protein synthesis-dependent mGluR-LTD at CA3-CA1 synapses.
Wu PY, Ji L, De Sanctis C, Francesconi A, Inglebert Y, McKinney RA. Wu PY, et al. PNAS Nexus. 2024 Feb 8;3(2):pgae062. doi: 10.1093/pnasnexus/pgae062. eCollection 2024 Feb. PNAS Nexus. 2024. PMID: 38384385 Free PMC article. - Fragile X syndrome: an update on developing treatment modalities.
Healy A, Rush R, Ocain T. Healy A, et al. ACS Chem Neurosci. 2011 Aug 17;2(8):402-10. doi: 10.1021/cn200019z. Epub 2011 Mar 22. ACS Chem Neurosci. 2011. PMID: 22860169 Free PMC article. Review. - Chemogenetic Activation of Excitatory Neurons Alters Hippocampal Neurotransmission in a Dose-Dependent Manner.
Pati S, Salvi SS, Kallianpur M, Vaidya B, Banerjee A, Maiti S, Clement JP, Vaidya VA. Pati S, et al. eNeuro. 2019 Nov 15;6(6):ENEURO.0124-19.2019. doi: 10.1523/ENEURO.0124-19.2019. Print 2019 Nov/Dec. eNeuro. 2019. PMID: 31645362 Free PMC article. - Roles of p75(NTR), long-term depression, and cholinergic transmission in anxiety and acute stress coping.
Martinowich K, Schloesser RJ, Lu Y, Jimenez DV, Paredes D, Greene JS, Greig NH, Manji HK, Lu B. Martinowich K, et al. Biol Psychiatry. 2012 Jan 1;71(1):75-83. doi: 10.1016/j.biopsych.2011.08.014. Epub 2011 Oct 5. Biol Psychiatry. 2012. PMID: 21978521 Free PMC article.
References
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–58. - PubMed
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous