Flavonoid composition of Citrus juices - PubMed (original) (raw)
Review
Flavonoid composition of Citrus juices
Giuseppe Gattuso et al. Molecules. 2007.
Abstract
In the early nineties the presence of flavonoids in Citrus juices began to attract the attention of a number of researchers, as a result of their biological and physiological importance. This short review will explore two different aspects. The first part will focus on analytical techniques for the characterization of juices from different Citrus fruits regarding their flavonoid content (even if present in only trace amounts), concentrating on the most widely used methods (LC-MS and LC-MS-MS). The second part analyzes data reported in the literature regarding the composition of Citrus juices. The main components that have been detected so far are flavanone-O-glycosides and flavone-O- or -C-glycosides. The presence of such derivatives in various hand-squeezed and industrial juices is discussed, with special emphasis on their correlation to different species.
Figures
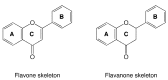
Figure 1
Flavone and flavanone skeletons.
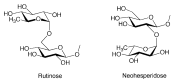
Figure 2
Rutinose and neohesperidose.
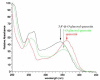
Figure 3
UV Spectra of quercetin (10, red trace), 3-_O_-glucosyl quercetin (green trace), and 3,4’-di-_O_-glucosyl quercetin (black trace).
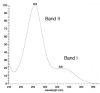
Figure 4
UV Spectrum of the flavanone hesperidin (23).
Figure 5
Negative mode (a) and positive mode (b) ESI-MS spectra of a flavanone bearing a disaccharide as a substituent (hesperidin, 23).
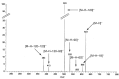
Figure 6
MS-MS spectrum in negative mode the aglycone hesperetin (1).
Figure 7
Negative mode MS-MS spectrum (focused on the pseudomolecular ion) of diosmetin 6,8-di-_C_-glucoside (32).
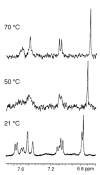
Figure 8
Variable temperature 1H-NMR spectrum of 6,8-di-_C_-glucosyl diosmetin (32), recorded in DMSO-_d_6 at 300 MHz. See ref. [33].
Figure 9
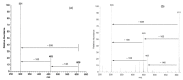
Simultaneously recorded DAD chromatograms of a red orange juice at two different wavelengths, for examples, at (a): 280 nm and (b): 325 nm.
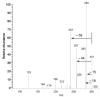
Figure 10
HPLC-DAD-ESI-MS-MS analysis of a lemon juice sample. a) Chromatogram at 280 nm; b) chromatogram at 325 nm; c) positive mode MS spectrum of the peak at RT 21.1 (labeled as diosmin 43 in the chromatograms); d) negative mode MS spectrum of 43; e) UV spectrum of 43; f) negative mode MS-MS focused on the m/e 607 ion.
Similar articles
- Simultaneous separation of flavanone glycosides and polymethoxylated flavones in citrus juices using liquid chromatography.
Mouly P, Gaydou EM, Auffray A. Mouly P, et al. J Chromatogr A. 1998 Mar 27;800(2):171-9. doi: 10.1016/s0021-9673(97)01131-x. J Chromatogr A. 1998. PMID: 9561761 - Separation of flavanone-7-O-glycoside diastereomers and analysis in citrus juices by multidimensional liquid chromatography coupled with mass spectrometry.
Aturki Z, Brandi V, Sinibaldi M. Aturki Z, et al. J Agric Food Chem. 2004 Aug 25;52(17):5303-8. doi: 10.1021/jf0400967. J Agric Food Chem. 2004. PMID: 15315361 - Flavonoid composition and antioxidant activity of juices from Chinotto ( Citrus x myrtifolia Raf.) fruits at different ripening stages.
Barreca D, Bellocco E, Caristi C, Leuzzi U, Gattuso G. Barreca D, et al. J Agric Food Chem. 2010 Mar 10;58(5):3031-6. doi: 10.1021/jf9044809. J Agric Food Chem. 2010. PMID: 20155909 - Vitamin C and the role of citrus juices as functional food.
Martí N, Mena P, Cánovas JA, Micol V, Saura D. Martí N, et al. Nat Prod Commun. 2009 May;4(5):677-700. Nat Prod Commun. 2009. PMID: 19445318 Review. - Citrus fruits--varieties, chemistry, technology, and quality evaluation. Part II. Chemistry, technology, and quality evaluation. A. Chemistry.
Ranganna S, Govindarajan VS, Ramana KV. Ranganna S, et al. Crit Rev Food Sci Nutr. 1983;18(4):313-86. doi: 10.1080/10408398309527366. Crit Rev Food Sci Nutr. 1983. PMID: 6354594 Review.
Cited by
- Genome-wide identification, classification, and characterization of lectin gene superfamily in sweet orange (Citrus sinensis L.).
Faysal Ahmed F, Dola FS, Zohra FT, Rahman SM, Konak JN, Sarkar MAR. Faysal Ahmed F, et al. PLoS One. 2023 Nov 13;18(11):e0294233. doi: 10.1371/journal.pone.0294233. eCollection 2023. PLoS One. 2023. PMID: 37956187 Free PMC article. - Innovative Processing Technologies to Develop a New Segment of Functional Citrus-Based Beverages: Current and Future Trends.
Vilas-Boas AA, Magalhães D, Campos DA, Porretta S, Dellapina G, Poli G, Istanbullu Y, Demir S, San Martín ÁM, García-Gómez P, Mohammed RS, Ibrahim FM, El Habbasha ES, Pintado M. Vilas-Boas AA, et al. Foods. 2022 Nov 29;11(23):3859. doi: 10.3390/foods11233859. Foods. 2022. PMID: 36496667 Free PMC article. Review. - Ultra-Performance Liquid Chromatography-Ion Mobility Separation-Quadruple Time-of-Flight MS (UHPLC-IMS-QTOF MS) Metabolomics for Short-Term Biomarker Discovery of Orange Intake: A Randomized, Controlled Crossover Study.
Lacalle-Bergeron L, Portolés T, López FJ, Sancho JV, Ortega-Azorín C, Asensio EM, Coltell O, Corella D. Lacalle-Bergeron L, et al. Nutrients. 2020 Jun 29;12(7):1916. doi: 10.3390/nu12071916. Nutrients. 2020. PMID: 32610451 Free PMC article. Clinical Trial. - Deciphering the crosstalk between inflammation and biofilm in chronic wound healing: Phytocompounds loaded bionanomaterials as therapeutics.
Sankar S, Kodiveri Muthukaliannan G. Sankar S, et al. Saudi J Biol Sci. 2024 Apr;31(4):103963. doi: 10.1016/j.sjbs.2024.103963. Epub 2024 Feb 23. Saudi J Biol Sci. 2024. PMID: 38425782 Free PMC article. Review. - Recent Trends in Potential Therapeutic Applications of the Dietary Flavonoid Didymin.
Yao Q, Lin MT, Zhu YD, Xu HL, Zhao YZ. Yao Q, et al. Molecules. 2018 Oct 6;23(10):2547. doi: 10.3390/molecules23102547. Molecules. 2018. PMID: 30301216 Free PMC article. Review.
References
- Harborne J. B. In: The Flavonoids. Chapman & Hall, editor. CRC; Boca Raton, FL: 1999. p. 66. Grotewold E. The Science of Flavonoids. Springer; New York, NY: 2006. Harborne J. B., Williams C. A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/S0031-9422(00)00235-1.
- Stafford H. A. Flavonoid Metabolism. CRC; Boca Raton, FL: 1990. pp. 1–59.
- Buslig B S., Manthey J. A., editors. Flavonoids in cell function. Kluwer Academic/Plenum Publishers; New York, NY: 2002. Forkmann G. Proc. 16th Int. Conf. Groupe Polyphenols. 1992;vol. 16:19–27. Lisbon. Herrmann K. Chem. Mikrobiol. Technol. Lebensm. 1970;12:161. Cody V., Middleton E., Harborne J. B., Beretz A., editors. Plant Flavonoids in Biology and Medicine. II: Biochemical, Cellular and Medicinal Properties. Progress in Clinical and Biological Research Vol. 280. Alan R. Liss; New York: 1988.
- Sharma D. K. Bioprospecting for drug, research and functional foods for the prevention of diseases – Role of flavonoids in drug development. J. Sci. Ind. Res. 2006;65:391–401. Cermak R., Wolffram S. The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms. Curr. Drug Metab. 2006;7:729–744. doi: 10.2174/138920006778520570. Wang H. K. The therapeutic potential of flavonoids. Expert Opin. Investig. Drugs. 2000;9:2103–2119. doi: 10.1517/13543784.9.9.2103. Di Carlo G., Mascolo N., Izzo A. A., Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65:337–353. doi: 10.1016/S0024-3205(99)00120-4. Manach C., Donovan J. L. Farmacokinetics and metabolism of dietary flavonoids in humans. Free Radical Res. 2004;38:771–785. Ortuno A., Gomez P., Baidez A., Frias V., Del Rio J. A. Citrus sp: a source of flavonoids of pharmaceutical interest. Potential Health Benefits of Citrus (ACS Symp. Ser. 936) 2006:175–185. Tapiero H., Tew K. D., Ba G.N., Mathe G. Polyphenols: do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 2002;56:200–207. doi: 10.1016/S0753-3322(02)00178-6.
- Valenzuela A., Sanhueza J., Nieto S. Natural antioxidants in functional foods: from food safety to health benefits. Grasas Aceites. 2003;54:295–303. Hooper L., Cassidy A. A review of the health care potential of bioactive compounds. J. Sci. Food Agric. 2006;86:1805–1813. doi: 10.1002/jsfa.2599.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases