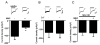Mind bomb-2 is an E3 ligase that ubiquitinates the N-methyl-D-aspartate receptor NR2B subunit in a phosphorylation-dependent manner - PubMed (original) (raw)
Mind bomb-2 is an E3 ligase that ubiquitinates the N-methyl-D-aspartate receptor NR2B subunit in a phosphorylation-dependent manner
Rachel Jurd et al. J Biol Chem. 2008.
Abstract
The N-methyl-D-aspartate receptor (NMDAR) plays a critical role in synaptic plasticity. Post-translational modifications of NMDARs, such as phosphorylation, alter both the activity and trafficking properties of NMDARs. Ubiquitination is increasingly being recognized as another post-translational modification that can alter synaptic protein composition and function. We identified Mind bomb-2 as an E3 ubiquitin ligase that interacts with and ubiquitinates the NR2B subunit of the NMDAR in mammalian cells. The protein-protein interaction and the ubiquitination of the NR2B subunit were found to be enhanced in a Fyn phosphorylation-dependent manner. Immunocytochemical studies reveal that Mind bomb-2 is localized to postsynaptic sites and colocalizes with the NMDAR in apical dendrites of hippocampal neurons. Furthermore, we show that NMDAR activity is down-regulated by Mind bomb-2. These results identify a specific E3 ubiquitin ligase as a novel interactant with the NR2B subunit and suggest a possible mechanism for the regulation of NMDAR function involving both phosphorylation and ubiquitination.
Figures
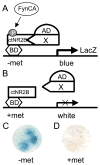
FIGURE 1. Yeast three-hybrid screen to identify Fyn phosphorylation-dependent NR2B interactants
Yeast were co-transformed with a rat brain cDNA library and a bait plasmid that expressed the cytoplasmic tail of the NR2B subunit of the NMDAR (ctNR2B) in-frame with a Gal4 DNA binding domain (BD) and a constitutively active form of Fyn (FynCA). FynCA expression was governed by a conditional methionine promoter, such that FynCA expression was induced in the absence of methionine (−met) (A) and repressed in the presence of methionine (+met) (B) in the yeast growth media. Colonies turning blue in the absence of methionine (C), but not in the presence of methionine (D), were identified as potential three-hybrid interactants reliant on the expression of FynCA.
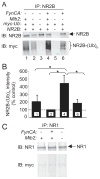
FIGURE 2. Mib2 interaction with the NR2B subunit of the NMDAR is enhanced in a Fyn phosphorylation-dependent manner
HEK293 cells were transiently transfected with plasmids encoding (A) the RING domain of Mib2 (HA-RING), and GFP-NR2B in the presence/absence of a plasmid encoding constitutively-active Fyn kinase (±FynCA), or (C) full-length Mib2 and GFP-NR2B in the presence/absence of FynCA. Immunoprecipitations (_IP_s) were carried out on total cell lysates (500 _μ_g) using anti-NR2B (goat) antibodies or IgG antibodies as control. Immune complexes were detected by Western blotting using anti-NR2B (rabbit), HA (rat), and phosphotyrosine (pY) antibodies. Inputs are 5% of total lysate. B and D, quantitation of the band intensity of RING or Mib2 binding in the presence of FynCA (normalized to NR2B IP levels) are plotted as mean ± S.E., compared with the amount of binding in the absence of FynCA. *, p < 0.05; n = 5; **, p < 0.01, n = 9 (one sample t test). E, HEK293 cells were transiently transfected with plasmids encoding HA-Mib2 and NR1. IPs were performed on total cell lysates (500 _μ_g) using anti-NR1 (mouse) antibodies and IgG (mouse) as control. Immune complexes were detected by Western blotting using anti-NR1 (mouse) and HA antibodies. Inputs are 5% of total lysate.
FIGURE 3. Mib2 interacts directly with NR2B
A, radiolabeled Mib2 (35S-Mib2) was generated by in vitro translation using full-length Mib2 cDNA. 35S-Mib binds to the cytoplasmic tail of maltose-binding protein-tagged NR2B (MBP-NR2B, lane 3), but not to MBP (lane 1) or to the cytoplasmic tail of the NR1 subunit (MBP-NR1, lane 2). It also binds to a phosphomimic mutant of NR2B (MBP-2B-mut, lane 4) in which the three main tyrosine residues for Fyn phosphorylation (Tyr1252, Tyr1336, Tyr1472) have been mutated to aspartic acid residues. 35S-Mib2 (10% of that used in the binding assay) was loaded as input. Samples were loaded in duplicate followed by Coomassie staining (bottom panel) to verify that equivalent amounts of MBP fusion proteins were used in the binding assays. B, deletion constructs of NR2B reveal that 35S-Mib binds to the distal part of the cytoplasmic tail of NR2B (MBP-2BΔN, lane 2) and less well to the proximal portion of the NR2B tail (MBP-2BΔC, lane 3). Coomassie-stained gel (bottom panel) demonstrates that equivalent amounts of MBP fusion proteins were used in the binding assays. C, quantitation of the binding of 35S-Mib to MBP-2BΔC compared with the binding of 35S-Mib to MBP-2BΔN (normalized to 100%). **, p < 0.01, n = 9 (one sample t test). D, schematic diagram illustrating the MBP-2BΔN (1086 –1482 aa) and MBP-2BΔC-(839 –1170) NR2B protein fragments that were expressed, as well as the sites of the three tyrosine residues that were mutated within the NR2B tail.
FIGURE 4. Identification of the domains of Mib2 that interact with the NR2B subunit
A, full-length Mib2 consists of a ZZ zinc finger domain (ZnF), ankyrin repeats (ANK), and RING finger domains (RING). Expression constructs consisting of regions encoding these domains were generated as indicated. B, HEK293 cells were transiently transfected with plasmids encoding GFP-NR2B and HA-tagged ZnF, ANK, and RING domains of Mib2. Immunoprecipitations (_IP_s) were carried out on total cell lysates (500 _μ_g) using anti-NR2B (lane 1) and IgG (lane 2) goat antibodies. Lane 3 depicts input samples (5% of total lysates). Immune complexes were detected by Western blotting using anti-NR2B (rabbit) and HA (rat) antibodies; n = 3.
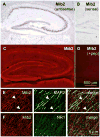
FIGURE 5. Mib2 is expressed in rat hippocampus
A and B, in situ hybridization using Mib2 (A) antisense and (B) sense probes in rat hippocampus. C–F, rat brain sections were immunostained using anti-Mib2 antibodies and (D) preincubation with Mib2 peptide antigen, or co-immunostained for (E) MAP2 or (F) NR1.
FIGURE 6. Mib2 is localized to postsynaptic sites in rat hippocampal neurons
Primary hippocampal cultures (21 days in vitro, d.i.v.) were immunostained for Mib2 and (A and B) PSD-95, (C) _α_-actinin or (D) MAP2.
FIGURE 7. Mib2 associates with the NR2B subunit in the hippocampus
Rat hippocampal slices were treated with vehicle (lane 1), with forskolin (10 μ
m
) for 15 min (lanes 2 and 3), or in the presence of KCl (50 m
m
) added at the last 5 min of forskolin treatment (lane 3). IPs were performed on homogenates with anti-NR2B (goat) antibodies and probed with anti-NR2B (goat), Mib2 (mouse) and phospho-tyrosine (pY) antibodies. Input is 5% of homogenate.
FIGURE 8. Mib2 ubiquitinates the NR2B subunit in a Fyn phosphorylation-dependent manner
A, HEK293 cells were transiently transfected with the following plasmids: FynCA, HA-Mib2, Myc-Ub, and GFP-NR2B. 48 h post-transfection, cells were treated with MG-132 (42 μ
m
) for 3 h. IPs were carried out on total cell lysates (500 _μ_g) using anti-NR2B (goat) antibodies. Immune complexes were detected by Western blotting using anti-NR2B (rabbit) and Myc antibodies to detect NR2B and NR2B-ubiquitinated species [NR2B-(Ub)_n_], respectively. B, quantification of the intensity of NR2B-(Ub)n normalized to NR2B-IP levels. Values are plotted as mean ± S.E., with the amount of NR2B-(Ub)n observed in the absence of FynCA and Mib2 (lane 2) taken to be 100%. *, p < 0.05 (one-way analysis of variance); n = 5 (lane 1), and 6 (all other lanes). C, HEK293 cells were transiently transfected with HA-Mib2, myc-Ub, NR1, and FynCA. 48 h post-transfection, cells were treated with MG-132 (40 μ
m
) for 3 h. IPs were carried out on total cell lysates (500 _μ_g) using anti-NR1 (mouse) antibodies. Immune complexes were detected by Western blotting using anti-NR1 (goat) and Myc antibodies to detect NR1 and NR1-ubiquitinated species, respectively. n = 3.
FIGURE 9. Expression of Mib2 depresses NR2B-containing NMDAR channel function in a ubiquitin-dependent manner
A, expression of Mib2 decreases the density of NMDA-elicited currents in HEK293 cells transfected with NR1 and NR2B subunits. The current density (pA/pF) was calculated as the peak amplitude of initial currents (pA) divided by the capacitance (pF) of cell membranes. Inset, sample traces of NMDA-evoked currents (5 s, 1 m
m
NMDA plus 50 μ
m
glycine); bar indicates NMDA application. Calibration, 2 s, 100 pA/pF. *, p < 0.05 (n = 13 and 12 cells for cells with and without Mib2 expression, respectively, Student’s t test). B, expression of a deletion mutant of Mib2 (ZnF) that does not bind NR2B does not affect NMDA-elicited currents (n = 12 and 11 for cells with and without ZnF expression, respectively). C, inhibition of the proteasome prevents the Mib2-induced decrease in the density of NMDA-elicited currents. HEK293 cells transfected with and without Mib2 were treated with the proteasome inhibitor, MG-132 (10 μ
m
), for 2–3 h before recordings (n = 14 and 13 for cells with and without Mib2, respectively).
Similar articles
- Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors.
Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Sanz-Clemente A, et al. Neuron. 2010 Sep 23;67(6):984-96. doi: 10.1016/j.neuron.2010.08.011. Neuron. 2010. PMID: 20869595 Free PMC article. - Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand.
Chung HJ, Huang YH, Lau LF, Huganir RL. Chung HJ, et al. J Neurosci. 2004 Nov 10;24(45):10248-59. doi: 10.1523/JNEUROSCI.0546-04.2004. J Neurosci. 2004. PMID: 15537897 Free PMC article. - Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems.
Wu HY, Hsu FC, Gleichman AJ, Baconguis I, Coulter DA, Lynch DR. Wu HY, et al. J Biol Chem. 2007 Jul 13;282(28):20075-87. doi: 10.1074/jbc.M700624200. Epub 2007 May 25. J Biol Chem. 2007. PMID: 17526495 Free PMC article. - Making of a Synapse: Recurrent Roles of Drebrin A at Excitatory Synapses Throughout Life.
Aoki C, Sherpa AD. Aoki C, et al. Adv Exp Med Biol. 2017;1006:119-139. doi: 10.1007/978-4-431-56550-5_8. Adv Exp Med Biol. 2017. PMID: 28865018 Review. - [Regulation of NMDA receptor function by Fyn-mediated tyrosine phosphorylation].
Nakazawa T, Tezuka T, Yamamoto T. Nakazawa T, et al. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002 Oct;22(5):165-7. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002. PMID: 12451687 Review. Japanese.
Cited by
- NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia.
Snyder MA, Gao WJ. Snyder MA, et al. Front Cell Neurosci. 2013 Mar 27;7:31. doi: 10.3389/fncel.2013.00031. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23543703 Free PMC article. - Mind Bomb-2 Regulates Hippocampus-dependent Memory Formation and Synaptic Plasticity.
Kim S, Kim T, Lee HR, Kong YY, Kaang BK. Kim S, et al. Korean J Physiol Pharmacol. 2015 Nov;19(6):515-22. doi: 10.4196/kjpp.2015.19.6.515. Epub 2015 Oct 16. Korean J Physiol Pharmacol. 2015. PMID: 26557018 Free PMC article. - Regulation of Synaptic NMDA Receptor Activity by Post-Translational Modifications.
Tahiri E, Corti E, Duarte CB. Tahiri E, et al. Neurochem Res. 2025 Mar 3;50(2):110. doi: 10.1007/s11064-025-04346-6. Neurochem Res. 2025. PMID: 40029461 Free PMC article. Review. - Calpain and the glutamatergic synapse.
Doshi S, Lynch DR. Doshi S, et al. Front Biosci (Schol Ed). 2009 Jun 1;1(2):466-76. doi: 10.2741/s38. Front Biosci (Schol Ed). 2009. PMID: 19482714 Free PMC article. Review. - The effects of proteasomal inhibition on synaptic proteostasis.
Hakim V, Cohen LD, Zuchman R, Ziv T, Ziv NE. Hakim V, et al. EMBO J. 2016 Oct 17;35(20):2238-2262. doi: 10.15252/embj.201593594. Epub 2016 Sep 9. EMBO J. 2016. PMID: 27613546 Free PMC article.
References
- Cull-Candy S, Brickley S, Farrant M. Curr Opin Neurobiol. 2001;11:327–335. - PubMed
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Annu Rev Pharmacol Toxicol. 2003;43:335–358. - PubMed
- Sheng M, Kim MJ. Science. 2002;298:776–780. - PubMed
- Suzuki T, Okumura-Noji K. Biochem Biophys Res Commun. 1995;216:582–588. - PubMed
- Yaka R, He DY, Phamluong K, Ron D. J Biol Chem. 2003;278:9630–9638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous