A cell cycle regulatory network controlling NF-kappaB subunit activity and function - PubMed (original) (raw)
A cell cycle regulatory network controlling NF-kappaB subunit activity and function
Benjamin Barré et al. EMBO J. 2007.
Retraction in
- Retraction: 'A cell cycle regulatory network controlling NF-κB subunit activity and function'.
Barré B, Perkins ND. Barré B, et al. EMBO J. 2014 Sep 1;33(17):1978. doi: 10.15252/embj.201470010. Epub 2014 Jul 28. EMBO J. 2014. PMID: 25069773 Free PMC article. No abstract available.
Abstract
Aberrantly active NF-kappaB complexes can contribute to tumorigenesis by regulating genes that promote the growth and survival of cancer cells. We have investigated NF-kappaB during the cell cycle and find that its ability to regulate the G1-phase expression of key proto-oncogenes is subject to regulation by the integrated activity of IkappaB kinase (IKK)alpha, IKKbeta, Akt and Chk1. The coordinated binding of NF-kappaB subunits to the Cyclin D1, c-Myc and Skp2 promoters is dynamic with distinct changes in promoter occupancy and RelA(p65) phosphorylation occurring through G1, S and G2 phases, concomitant with a switch from coactivator to corepressor recruitment. Akt activity is required for IKK-dependent phosphorylation of NF-kappaB subunits in G1 and G2 phases, where Chk1 is inactive. However, in S-phase, Akt is inactivated, while Chk1 phosphorylates RelA and associates with IKKalpha, inhibiting the processing of the p100 (NF-kappaB2) subunit, which also plays a critical role in the regulation of these genes. These data reveal a complex regulatory network integrating NF-kappaB with the DNA-replication checkpoint and the expression of critical regulators of cell proliferation.
Figures
Figure 1
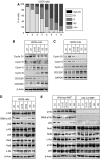
p100 processing to p52 is inhibited in S phase. (A) U2OS cells were fractionated by centrifugal elutriation and stained with propidium iodide for analysis by FACS. A representative distribution of cells at different cell cycle stages is shown. Fractions designated G2 will contain some cells undergoing mitosis. However, since only adherent cells were harvested, most mitotic cells are lost. The enrichment of S-phase fractions may be underestimated as cells in early and late S phase may be scored as G1 or G2 phases, respectively. Cell cycle analysis of elutriated fraction used in all experiments can be found in Supplementary Figure 9. (B) Fractionated U2OS whole-cell protein extracts were pooled according to cell cycle stage and subjected to western blot analysis using antibodies to Cyclin D1, E, A and B1, CDC25A and CDC25C. (C) RT–PCR analysis was performed using primers specific to Cyclin D1, E, A, CDC25C and GAPDH control, using total RNA prepared from U2OS cells following centrifugal elutriation. (D, E) Whole-cell extracts prepared from U2OS (D) or w/t and p52/100-null MEF (E) from the indicated cell cycle stages were subjected to western blot analysis using antibodies to the indicated NF-κB subunits.
Figure 2
Cell cycle expression of Cyclin D1, c-Myc and Skp2. (A) U2OS whole-cell extracts, from each cell cycle stage, were subjected to western blot analysis using antibodies to Cyclin D1, Skp2 and c-Myc. (B) Cyclin D1, Skp2 and c-Myc are preferentially expressed in G1 phase. Quantitative PCR analysis was performed using primers specific to Cyclin D1, Skp2 and c-Myc and total RNA prepared from U2OS cells following centrifugal elutriation. (C–E) p52 regulates Cyclin D1, Skp2 and c-Myc expression. U2OS cells were transfected with an siRNA targeting p52/p100. Protein (C) and mRNA (D, E) expression were analyzed as shown. In (D), analysis was performed using quantitative, real-time PCR. In these and subsequent experiments, cells were transfected with siRNA on day 1 and harvested on day 4.
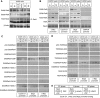
Figure 3
Cell cycle-dependent binding of NF-κB subunits to the Cyclin D1, c-Myc and Skp2 promoters. (A, B) Analysis of NF-κB subunit, coactivator and corepressor recruitment to the promoters of NF-κB target genes in U2OS cells. ChIP analysis of the Cyclin D1, Skp2, c-Myc and GAPDH control promoters (A), or IκBα, Bcl-xL and Bcl-2 promoters (B) was performed using antibodies to the indicated proteins in U2OS cells following centrifugal elutriation. Further details on ChIP primers used in U2OS cell experiments can be found in Supplementary Figure 10. (C) Table summarizing ChIP assay data from Figure 3A and Supplementary Figure 3 describing NF-κB subunit and coactivator/corepressor binding to the Cyclin D1, c-Myc and Skp2 promoters.
Figure 4
Different NF-κB homodimer and heterodimer complexes are present on Cyclin D1, c-Myc and Skp2 promoters in G1 and G2 phases. (A) NF-κB subunits were immunoprecipitated from whole-cell lysates prepared from asynchronous cells and analyzed by western blotting as indicated. (B) The effect of depleting p52 on protein binding to the Cyclin D1, Skp2 and c-Myc promoters. U2OS cells were transfected with an siRNA targeting p52/p100. Cyclin D1, Skp2, c-Myc and GAPDH promoters were analyzed by using ChIP and Q-PCR as shown. (C) Distinct NF-κB subunit complexes bind the Cyclin D1, Skp2 and c-Myc promoters. ReChIP analysis of the Cyclin D1, Skp2 and c-Myc promoters was performed using the indicated antibodies. (D) Table summarizing ChIP and ReChIP assay data from (B) and (C) and Supplementary Figure 4 describing the co-occupancy of the Cyclin D1, c-Myc and Skp2 promoters by different NF-κB subunits and coactivator/corepressors.
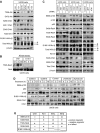
Figure 5
RelA phosphorylation changes during the cell cycle. (A) Whole-cell lysates were prepared from U2OS cells following centrifugal elutriation and immunoblotted for RelA S468 and S536 phosphorylation and β-actin. RelA was immunoprecipitated before western blot analysis for RelA T505 phosphorylation. (B) ChIP analysis of S468-, T505- and S536-phosphorylated RelA recruitment to the Cyclin D1, Skp, c-Myc and GAPDH promoters in each cell cycle stage of U2OS cells. (C, D) S468-, T505- and S536-phosphorylated RelA differentially associate with coactivators and corepressors at different stages of the cell cycle. ReChIP analysis of the Cyclin D1, Skp2 and c-Myc gene promoters using elutriated U2OS cells was performed using the indicated combinations of antibodies. (E) Table summarizing ChIP and ReChIP assay data from (C) and (D) describing the co-occupancy of the Cyclin D1, c-Myc and Skp2 promoters by differentially phosphorylated forms of RelA.
Figure 6
RelA phosphorylation in G1 and G2 phases of the cell cycle is IKK dependent. (A) Asynchronous U2OS cells were treated with the Bay11-7082 and IKKiIV ([5-(p-Fluorophenyl)-2-ureido]thiophene-3-carboxamide) IKK inhibitors, for the indicated times. Whole-cell lysates were prepared and immunoblotted for S468- and S536-phosphorylated RelA. (B) Whole-cell extracts from wild-type and IKKα- or IKKβ-null MEFs from each cell cycle stages were subjected to western blot analysis using antibodies to phosphorylated RelA and p100. (C) U2OS cells were transfected with siRNAs targeting IKKα or IKKβ whole-cell lysates were prepared and analyzed by western blotting as shown.
Figure 7
Antagonistic regulation of NF-κB by Akt1 and Chk1. (A) Akt and Chk1 activity fluctuates during the cell cycle. Whole cells extracts from elutriated U2OS cells were subjected to western blot analysis using antibodies to phosphorylated Akt, Chk1 and IKKα/β. (B) Chk1 was immunoprecipitated from whole-cell extracts prepared from elutriated U2OS cells. The immunoprecipitate was incubated with whole-cell lysate from serum-starved U2OS cells. The lysates were analyzed by western blotting with antibodies to T505-phosphorylated RelA and total RelA. (C) U2OS cells were transfected with siRNAs targeting Akt1, Akt2, Chk1, Chk2, ATR and ATM in U2OS cells. Whole-cell lysates were prepared and analyzed by western blotting with antibodies to the indicated phosphorylated NF-κB and IKK subunits. Note, the basal level of some bands can be seen varying between panels. This is due to selection of different gel exposures to best allow visualization of induction or repression of signal strength. All experiments shown were derived from the same set of protein extracts, although separate gels were required to generate the complete data set. (D) Asynchronous U2OS cells were treated with caffeine and Gö6976, which inhibit ATR and Chk1, respectively, aphidicolin that causes S-phase arrest leading to an activation of Chk1, or LY294002, an inhibitor of PI-3 kinase activity and the Akt pathway, for the indicated times. Whole-cell lysates were prepared and immunoblotted for S866-phosphorylated p100, S180/1-phosphorylated IKKα/β, S345-phosphorylated Chk1 and total p100. (E) Table summarizing the regulation of different markers of NF-κB pathway activity by the Akt1, Chk1 and IKK kinases.
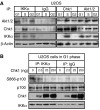
Figure 8
Chk1 is associated with and inhibits IKKα activity. (A) IKKα associates with Chk1 in S phase. IKKα, Akt1/2 and Chk1 were immunoprecipitated from whole-cell extracts prepared from elutriated U2OS cells. The immunoprecipitates were resolved by SDS–PAGE before western blotting with antibodies to IKKα, Chk1 or Akt1/2. A β-actin western blot from the input material is also shown. (B) Chk1 inhibits IKKα activity in vitro. IKKα was immunoprecipitated from G1-phase-enriched extract prepared from elutriated U2OS cells. The immunoprecipitate was incubated with whole-cell lysate from serum-starved U2OS cells with or without the indicated quantities of recombinant Chk1. These lysates were then analyzed by western blotting with antibodies to S866-phosphorylated p100, total Chk1, total p100 and IKKα.
Figure 9
Chk1 and Akt1 antagonistically regulate Cyclin D1, c-Myc and Skp2 activity. (A, B) The expression of Cyclin D1, Skp2 and c-Myc expression is regulated by Akt1 and Chk1. Quantitative PCR analysis (A) or western blot analysis (B) was performed on extracts prepared from asynchronous U2OS cells treated with the indicated siRNAs. (C) Analysis of p52 NF-κB, HDAC1 and CBP recruitment to the promoters of NF-κB target genes in each cell cycle stage following Chk1 and Akt pathway inhibition. ChIP analysis of the Cyclin D1, Skp2, c-Myc and GAPDH promoters was performed in U2OS cells following centrifugal elutriation of U2OS cells with or without Gö6976 or LY294002 treatment. Analysis of the other NF-κB subunits is shown in Supplementary Figure 8. (D, E) RNA and whole-cell protein extracts were prepared from elutriated U2OS cells treated with Gö6976 or LY294002 and subjected to quantitative PCR (D) or western blot (E) analysis.
Figure 10
Model depicting the integration of NF-κB activity with the Akt1, IKK and Chk1 pathways during the cell cycle. During G1 and G2 phases, Akt activity is required for IKKα and β activity, resulting in the processing of p100 to p52 and the phosphorylation of RelA at serines 468 or 536. At the same time, Akt acts to inhibit Chk1, by phosphoylating it at serine 280. However, in S phase, Chk1 becomes activated and Akt activity is suppressed. Chk1 becomes directly associated with IKKα, inhibiting its activity. As a consequence, processing of p100 to p52 is inhibited. Furthermore, RelA becomes phosphorylated at Thr505, resulting in recruitment of HDAC1 to the Cyclin D1, c-Myc and Skp2 promoters. In G2 phase, NF-κB-mediated suppression of these genes is maintained, an effect associated with the recruitment of additional NF-κB complexes containing p50, RelB and c-Rel, even in the presence of active Akt and inhibited Chk1, suggesting an additional regulatory mechanism.
Similar articles
- Regulation and function of IKK and IKK-related kinases.
Häcker H, Karin M. Häcker H, et al. Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review. - Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38.
Madrid LV, Mayo MW, Reuther JY, Baldwin AS Jr. Madrid LV, et al. J Biol Chem. 2001 Jun 1;276(22):18934-40. doi: 10.1074/jbc.M101103200. Epub 2001 Mar 20. J Biol Chem. 2001. PMID: 11259436 - Regulation of p53 tumour suppressor target gene expression by the p52 NF-kappaB subunit.
Schumm K, Rocha S, Caamano J, Perkins ND. Schumm K, et al. EMBO J. 2006 Oct 18;25(20):4820-32. doi: 10.1038/sj.emboj.7601343. Epub 2006 Sep 21. EMBO J. 2006. PMID: 16990795 Free PMC article. - Regulation of I(kappa)B kinase complex by phosphorylation of (gamma)-binding domain of I(kappa)B kinase (beta) by Polo-like kinase 1.
Higashimoto T, Chan N, Lee YK, Zandi E. Higashimoto T, et al. J Biol Chem. 2008 Dec 19;283(51):35354-67. doi: 10.1074/jbc.M806258200. Epub 2008 Oct 27. J Biol Chem. 2008. PMID: 18957422 Free PMC article. - Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.
Karin M, Ben-Neriah Y. Karin M, et al. Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
- Inductive effect of phytoglycoprotein (38 kDa) on G₀/G₁ arrest and apoptosis in diethylnitrosamine-treated ICR mice.
Lee J, Lim KT. Lee J, et al. Mol Cell Biochem. 2013 Mar;375(1-2):31-8. doi: 10.1007/s11010-012-1524-3. Epub 2012 Dec 2. Mol Cell Biochem. 2013. PMID: 23212447 - Molecular Targets in Hepatocarcinogenesis and Implications for Therapy.
Wu MY, Yiang GT, Cheng PW, Chu PY, Li CJ. Wu MY, et al. J Clin Med. 2018 Aug 13;7(8):213. doi: 10.3390/jcm7080213. J Clin Med. 2018. PMID: 30104473 Free PMC article. Review. - IKK-dependent, NF-κB-independent control of autophagic gene expression.
Comb WC, Cogswell P, Sitcheran R, Baldwin AS. Comb WC, et al. Oncogene. 2011 Apr 7;30(14):1727-32. doi: 10.1038/onc.2010.553. Epub 2010 Dec 13. Oncogene. 2011. PMID: 21151171 Free PMC article. - Glycogen synthase kinase (GSK)-3 and the double-strand RNA-dependent kinase, PKR: When two kinases for the common good turn bad.
Piazzi M, Bavelloni A, Faenza I, Blalock W. Piazzi M, et al. Biochim Biophys Acta Mol Cell Res. 2020 Oct;1867(10):118769. doi: 10.1016/j.bbamcr.2020.118769. Epub 2020 Jun 5. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32512016 Free PMC article. Review. - Cyclin-dependent kinase 6 phosphorylates NF-κB P65 at serine 536 and contributes to the regulation of inflammatory gene expression.
Buss H, Handschick K, Jurrmann N, Pekkonen P, Beuerlein K, Müller H, Wait R, Saklatvala J, Ojala PM, Schmitz ML, Naumann M, Kracht M. Buss H, et al. PLoS One. 2012;7(12):e51847. doi: 10.1371/journal.pone.0051847. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300567 Free PMC article.
References
- Albanese C, D'Amico M, Reutens AT, Fu M, Watanabe G, Lee RJ, Kitsis RN, Henglein B, Avantaggiati M, Somasundaram K, Thimmapaya B, Pestell RG (1999) Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J Biol Chem 274: 34186–34195 - PubMed
- Bromberg JF, Fan Z, Brown C, Mendelsohn J, Darnell JE Jr (1998) Epidermal growth factor-induced growth inhibition requires Stat1 activation. Cell Growth Differ 9: 505–512 - PubMed
- Campbell KJ, Rocha S, Perkins ND (2004) Active repression of antiapoptotic gene expression by RelA(p65) NF-κB. Mol Cell 13: 853–865 - PubMed
- Campbell KJ, Witty JM, Rocha S, Perkins ND (2006) Cisplatin mimics ARF tumor suppressor regulation of RelA (p65) nuclear factor-κB transactivation. Cancer Res 66: 929–935 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous