Repression by Groucho/TLE/Grg proteins: genomic site recruitment generates compacted chromatin in vitro and impairs activator binding in vivo - PubMed (original) (raw)
Repression by Groucho/TLE/Grg proteins: genomic site recruitment generates compacted chromatin in vitro and impairs activator binding in vivo
Takashi Sekiya et al. Mol Cell. 2007.
Abstract
Groucho-related (Gro/TLE/Grg) corepressors meditate embryonic segmentation, dorsal-ventral patterning, neurogenesis, and Notch and Wnt signaling. Although Gro/TLE/Grgs disrupt activator complexes and recruit histone deacetylases (HDAC), activator complexes can be disrupted in various ways, HDAC recruitment does not account for full corepressor activity, and a direct role for Gro/TLE/Grg binding and altering chromatin structure has not been explored. Using diverse chromatin substrates in vitro, we show that Grg3 creates higher-order, condensed complexes of polynucleosome arrays. Surprisingly, such complexes are in an open, exposed configuration. We find that chromatin binding enables Grg3 recruitment by a transcription factor and the creation of a closed, poorly accessible domain spanning three to four nucleosomes. Targeted recruitment of Grg3 blankets a similar-sized region in vivo, impairing activator recruitment and repressing transcription. These activities of a Groucho protein represent a newly discovered mechanism which differs from that of other classes of corepressors.
Figures
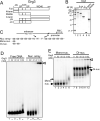
Fig. 1. Grg3 binding to nucleosomal substrates
(A) Deletion mutant series of Grg3. 4N, tetramerization domain; GP, glycine/proline rich; CcN, phosphorylation sites, nuclear localization; SP, serine/proline rich; WD40, repeat domain. (B) SDS-PAGE of purified Grg3 proteins. (C) alb1 gene and nucleosome templates (McPherson et al., 1993; Shim et al., 1998; Cirillo and Zaret, 1999). 5S: 5S rDNA. (D) EMSA with end-labeled alb1 enhancer-containing nucleosome arrays or free DNA; unbound DNA or nucleosome arrays at bottom of agarose gel. Complexes of Grg3 and arrays were retained in wells; open arrowheads. (E) EMSA with 5S rDNA mono- and dinucleosomes; polyacrylamide gel. Unbound probes; black arrowheads. Grg3 complexes with nucleosomal DNA, black arrows. The Grg3/dinucleosome complex was also detected at the wells; open arrowhead.
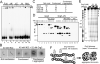
Fig. 2. Condensation and aggregation of nucleosome arrays by Grg3
(A) EMSA of designated Grg3 variant proteins and arrays. Arrowhead, complexes retained in the wells. (B) Electron microscopic analysis. Complexes were classified into categories indicated in (C). 50 arrays were analyzed for each Grg3 protein. (D) Conformation change of Grg3 upon interaction with arrays. Full- and ΔN-Grg3 proteins were digested with V8 protease and the Grg3 products were detected by Western blotting. Association of Grg3 with arrays induced V8 cleavage (asterisk), which is also detected with ΔN-Grg3 regardless of array addition (arrowhead). Open arrowhead, undigested Grg3. (E) DNaseI digestion of Grg3 binding to the arrays. Positions of nucleosomes on reconsitituted arrays are indicated to left of the gel. N1, N2, and P correspond to the alb1 enhancer N1, N2 sequences, and minimal promoter, respectively. Digestion was with 35 or 70 ng DNaseI. (F) Upon binding to chromatin Grg3 changes its conformation, and now both condenses chromatin and forms aggregates.
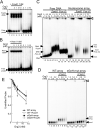
Fig. 3. FoxA1 recruitment of Grg3 to arrays, but not free DNA
(A), (B) Absence of FoxA1-Grg3 and Hes1-Grg3 complexes on free DNA. End-labeled probes containing a FoxA1 (Liu et al., 1991) or Hes1 binding site (Lee et al., 2001) were incubated with FoxA1 or Hes1 (lane 2), Grg3 (lanes 7−10), or both Grg3 and FoxA1 or Hes1 (lanes 3−6). Note the absence of supershifting of FoxA1-or Hes1-DNA complex by Grg3. (C, D) FoxA1 enhances Grg3-array interactions, but not Grg3-DNA interactions (see text). The positions of unshifted DNA, arrays, and complexes are noted by black arrowheads. Complexes with Grg3 were primarily at wells (open arrowhead). (E) Quantitation of PhosphorImager data as in (D). n=4, *P < 0.05 for FoxA1 and Grg3 with wt arrays vs with eGeH site mutant arrays.
Fig. 4. Protection of 3−4 local nucleosomes by FoxA1 recruitment of Grg3
(A) DNaseI digestion (35 or 70 ng) of Grg3 and FoxA1 binding to arrays. Lanes 14−17, FoxA1 binding site mutant arrays. (B) Accessibility of restriction enzymes to arrays with or without Grg3 and FoxA1. Arrays were digested with either XbaI or EcoRI with or without Grg3 and 20 nM FoxA1. Each band (E1−8, X) labeled on right is shown in (C). (C) Quantification of the results from 3 experiments as in (B). Results of arrays alone were normalized to a value of 1 (black bars), and relative cleavage in the presence of proteins, at each site, is shown (colored bars). (D) EcoRI digestion of Grg3 and Hes1 binding to the Hes1-arrays. Arrays were digested with EcoRI with 52 nM of Grg3 (lanes 2, 4) and 40 nM of Hes1 (lanes 3, 4). The position of the nucleosome with Hes1 binding sites is denoted by 'H'.
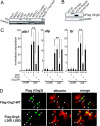
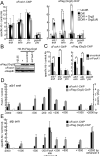
Fig. 5. Grg3 inhibits FoxA target gene expression in mouse and human cells
(A) Grg protein expression in cell lines was analyzed by Western blotting with the indicated antibodies. (B) Western blotting of whole cell extracts of H2.35, infected with Flag-Grg3-full, Flag-Grg3-ΔN, and GFP retroviruses as shown. (C) Effect of full- and ΔN-Grg3, and GFP expression on alb1,afp, and ttr mRNA levels in H2.35, normalized to both actin and hprt. Results with mock-infected, undifferentiated samples were set to 1; n = 3. *p <0.05, **p <0.005 for designated conditions. (D) HepG2 were infected lentiviruses expressing Flag-Grg3 or Flag-Grg3-L36D/L85D mutant. Flag-tag (left) and albumin (right) immunofluorescences were merged in the right panels. Arrowheads denote cells expressing exogenous Flag-Grg3. All cells in the field are evident.
Fig. 6. FoxA1 recruits Grg3 to blanket local enhancer regions
(A) ChIP were performed on mock-infected undifferentiated H2.35, mock-infected, Flag-Grg3, and Flag-Grg3-ΔN retrovirus-infected, differentiated H2.35 cells with FoxA1 and Flag antibodies. The ratio between the scores obtained with the specific antibodies and control antibodies are shown with SD. (B) H2.35 expressing Flag-Grg3, with or without siRNA, were incubated for 96 hr at 37°C. Flag-Grg3, FoxA1, and Gapdh proteins were detected by Western blotting. (C) Depletion of FoxA1 decreased the binding of both FoxA1 and Grg3 to the alb1 and afp enhancers. H2.35 expressing Flag-Grg3 were transfected with control- or FoxA1-siRNA, incubated for 96 hr at 37°C, and subjected to ChIP. (D, E) FoxA1 localizes to its target sites, but Grg3 spreads locally from the FoxA1 recruitment site. ChIP were performed on Flag-Grg3-expressing, differentiated H2.35. ChIP products were analyzed with primer sets at the indicated distances from the FoxA1 sites at the alb1 enhancer (D) and afp enhancers III and II (E). n = 3. *p <0.05, *p <0.005.
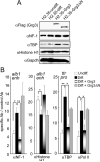
Fig. 7. FoxA-recruited Grg3 impairs binding by transcriptional activators
(A) Western blotting of whole cell lysates showing that ectopic expression of Grg3 isoforms does not reduce the levels of NF-1, TBP, or histone H1. (B) ChIP data (n=3) for the designated proteins at the alb1 enhancer or ttr promoter; pol II, anti-phosphorylated serine 5 of the C-terminal repeat. The data show that NF-1, TBP, and pol II exhibit enhanced recruitment to the designated regulatory elements during H2.35 cell differentiation (black bars) and that this recruitment is blocked by prior binding of Grg3 (graded bars); Grg3-deltaN had no effect (grey bars). *p <0.05, **p <0.005.
Similar articles
- Groucho binds two conserved regions of LEF-1 for HDAC-dependent repression.
Arce L, Pate KT, Waterman ML. Arce L, et al. BMC Cancer. 2009 May 21;9:159. doi: 10.1186/1471-2407-9-159. BMC Cancer. 2009. PMID: 19460168 Free PMC article. - Role for Hes1-induced phosphorylation in Groucho-mediated transcriptional repression.
Nuthall HN, Husain J, McLarren KW, Stifani S. Nuthall HN, et al. Mol Cell Biol. 2002 Jan;22(2):389-99. doi: 10.1128/MCB.22.2.389-399.2002. Mol Cell Biol. 2002. PMID: 11756536 Free PMC article. - Molecular functions of the TLE tetramerization domain in Wnt target gene repression.
Chodaparambil JV, Pate KT, Hepler MR, Tsai BP, Muthurajan UM, Luger K, Waterman ML, Weis WI. Chodaparambil JV, et al. EMBO J. 2014 Apr 1;33(7):719-31. doi: 10.1002/embj.201387188. Epub 2014 Mar 3. EMBO J. 2014. PMID: 24596249 Free PMC article. - Groucho/TLE family proteins and transcriptional repression.
Chen G, Courey AJ. Chen G, et al. Gene. 2000 May 16;249(1-2):1-16. doi: 10.1016/s0378-1119(00)00161-x. Gene. 2000. PMID: 10831834 Review. - Mammalian Groucho homologs: redundancy or specificity?
Gasperowicz M, Otto F. Gasperowicz M, et al. J Cell Biochem. 2005 Jul 1;95(4):670-87. doi: 10.1002/jcb.20476. J Cell Biochem. 2005. PMID: 15861397 Review.
Cited by
- Sex determination in Drosophila: The view from the top.
Salz HK, Erickson JW. Salz HK, et al. Fly (Austin). 2010 Jan-Mar;4(1):60-70. doi: 10.4161/fly.4.1.11277. Epub 2010 Jan 21. Fly (Austin). 2010. PMID: 20160499 Free PMC article. Review. - TLE3 Sustains Luminal Breast Cancer Lineage Fidelity to Suppress Metastasis.
Anstine LJ, Majmudar PR, Aponte A, Singh S, Zhao R, Weber-Bonk KL, Abdul-Karim FW, Valentine M, Seachrist DD, Grennel-Nickelson KE, Cuellar-Vite L, Sizemore GM, Sizemore ST, Webb BM, Thompson CL, Keri RA. Anstine LJ, et al. Cancer Res. 2023 Apr 4;83(7):997-1015. doi: 10.1158/0008-5472.CAN-22-3133. Cancer Res. 2023. PMID: 36696357 Free PMC article. - Roles of Non-Canonical Wnt Signalling Pathways in Bone Biology.
Lojk J, Marc J. Lojk J, et al. Int J Mol Sci. 2021 Oct 7;22(19):10840. doi: 10.3390/ijms221910840. Int J Mol Sci. 2021. PMID: 34639180 Free PMC article. Review. - The yin and yang of pioneer transcription factors: Dual roles in repression and activation.
Katsuda T, Sussman JH, Zaret KS, Stanger BZ. Katsuda T, et al. Bioessays. 2024 Oct;46(10):e2400138. doi: 10.1002/bies.202400138. Epub 2024 Jul 26. Bioessays. 2024. PMID: 39058903 - Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer.
Sahu B, Laakso M, Ovaska K, Mirtti T, Lundin J, Rannikko A, Sankila A, Turunen JP, Lundin M, Konsti J, Vesterinen T, Nordling S, Kallioniemi O, Hautaniemi S, Jänne OA. Sahu B, et al. EMBO J. 2011 Sep 13;30(19):3962-76. doi: 10.1038/emboj.2011.328. EMBO J. 2011. PMID: 21915096 Free PMC article.
References
- Bossard P, Zaret KS. Repressive and restrictive mesodermal interactions with gut endoderm: possible relation to Meckel's Diverticulum. Development. 2000;127:4915–4923. - PubMed
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998;395:604–608. - PubMed
- Chaya D, Hayamizu T, Bustin M, Zaret KS. Transcription factor FoxA (HNF3) on a nucleosome at an enhancer complex in liver chromatin. J Biol Chem. 2001;276:44385–44389. - PubMed
- Chen G, Courey AJ. Groucho/TLE family proteins and transcriptional repression. Gene. 2000;249:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM047903/GM/NIGMS NIH HHS/United States
- R37 GM036477/GM/NIGMS NIH HHS/United States
- R37 GM036477-23/GM/NIGMS NIH HHS/United States
- R01 GM47903/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources