Salivary proteomic and genomic biomarkers for primary Sjögren's syndrome - PubMed (original) (raw)
. 2007 Nov;56(11):3588-600.
doi: 10.1002/art.22954.
Jianghua Wang, Jiska Meijer, Sonya Ieong, Yongming Xie, Tianwei Yu, Hui Zhou, Sharon Henry, Arjan Vissink, Justin Pijpe, Cees Kallenberg, David Elashoff, Joseph A Loo, David T Wong
Affiliations
- PMID: 17968930
- PMCID: PMC2856841
- DOI: 10.1002/art.22954
Salivary proteomic and genomic biomarkers for primary Sjögren's syndrome
Shen Hu et al. Arthritis Rheum. 2007 Nov.
Abstract
Objective: To identify a panel of protein and messenger RNA (mRNA) biomarkers in human whole saliva (WS) that may be used in the detection of primary Sjögren's syndrome (SS).
Methods: Mass spectrometry and expression microarray profiling were used to identify candidate protein and mRNA biomarkers of primary SS in WS samples. Validation of the discovered mRNA and protein biomarkers was also demonstrated using real-time quantitative polymerase chain reaction and immunoblotting techniques.
Results: Sixteen WS proteins were found to be down-regulated and 25 WS proteins were found to be up-regulated in primary SS patients compared with matched healthy control subjects. These proteins reflected the damage of glandular cells and inflammation of the oral cavity system in patients with primary SS. In addition, 16 WS peptides (10 up-regulated and 6 down-regulated in primary SS) were found at significantly different levels (P < 0.05) in primary SS patients and controls. Using stringent criteria (3-fold change; P < 0.0005), 27 mRNA in saliva samples were found to be significantly up-regulated in the primary SS patients. Strikingly, 19 of 27 genes that were found to be overexpressed were interferon-inducible or were related to lymphocyte filtration and antigen presentation known to be involved in the pathogenesis of primary SS.
Conclusion: Our preliminary study has indicated that WS from patients with primary SS contains molecular signatures that reflect damaged glandular cells and an activated immune response in this autoimmune disease. These candidate proteomic and genomic biomarkers may improve the clinical detection of primary SS once they have been further validated. We also found that WS contains more informative proteins, peptides, and mRNA, as compared with gland-specific saliva, that can be used in generating candidate biomarkers for the detection of primary SS.
Figures
Figure 1
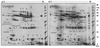
Comparative analysis of proteins in whole saliva (WS) samples from patients with primary Sjögren’s syndrome (pSS) and age-, sex-, and ethnicity-matched control subjects, as determined by 2-dimensional gel electrophoresis (2-DE) and liquid chromatography–quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS). Shown are the 2-DE patterns of proteins in pooled WS from 10 control subjects and 10 primary SS patients. A total of 100 µg of total proteins from each pooled sample was used for the 2-D gel separation. The differentially expressed proteins (spots 1–42; see Table 1 for the complete list) were identified using in-gel tryptic digestion and LC-Q-TOF-MS.
Figure 2
Analysis of α-enolase by electrospray ionization tandem mass spectrometry (ESI-MS/MS) and immunoblotting. A, ESI-MS/MS spectrum of the tryptic peptide TIAPALVSK (mass/charge [m/z] 450.3 atomic mass units [amu]) from α-enolase. This protein was found to be overexpressed in whole saliva from patients with primary Sjögren’s syndrome (pSS), as determined by 2-dimensional gel electrophoresis. B, Immunoblotting of whole saliva from 10 patients with primary SS and 10 age-, sex-, and ethnicity-matched control subjects for α-enolase and actin. An equal amount of proteins from each sample was used for the immunoblots.
Figure 3
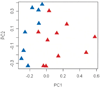
Principal components analysis of the gene expression data in patients with primary Sjögren’s syndrome (SS) and in age-, sex-, and ethnicity-matched control subjects. Results of the principal components (PC1 and PC2) analysis suggest that the gene expression data we obtained segregated the 8 control subjects (blue symbols) from the 10 primary SS patients (red symbols).
Figure 4
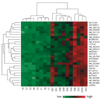
Heat map of 27 mRNA that were significantly up-regulated in patients with primary Sjögren’s syndrome (SS) as compared with the age-, sex-, and ethnicity-matched control subjects, as determined by microarray profiling analysis. Control and SS patient numbers are shown at the bottom.
Similar articles
- Proteomic and histopathological characterisation of sicca subjects and primary Sjögren's syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers.
Aqrawi LA, Galtung HK, Guerreiro EM, Øvstebø R, Thiede B, Utheim TP, Chen X, Utheim ØA, Palm Ø, Skarstein K, Jensen JL. Aqrawi LA, et al. Arthritis Res Ther. 2019 Jul 31;21(1):181. doi: 10.1186/s13075-019-1961-4. Arthritis Res Ther. 2019. PMID: 31366407 Free PMC article. - Proteomic analysis of saliva: a unique tool to distinguish primary Sjögren's syndrome from secondary Sjögren's syndrome and other sicca syndromes.
Baldini C, Giusti L, Ciregia F, Da Valle Y, Giacomelli C, Donadio E, Sernissi F, Bazzichi L, Giannaccini G, Bombardieri S, Lucacchini A. Baldini C, et al. Arthritis Res Ther. 2011;13(6):R194. doi: 10.1186/ar3523. Epub 2011 Nov 25. Arthritis Res Ther. 2011. PMID: 22117835 Free PMC article. - Preclinical validation of salivary biomarkers for primary Sjögren's syndrome.
Hu S, Gao K, Pollard R, Arellano-Garcia M, Zhou H, Zhang L, Elashoff D, Kallenberg CG, Vissink A, Wong DT. Hu S, et al. Arthritis Care Res (Hoboken). 2010 Nov;62(11):1633-8. doi: 10.1002/acr.20289. Epub 2010 Jul 8. Arthritis Care Res (Hoboken). 2010. PMID: 20617533 Free PMC article. - Salivary Biomarkers in Patients with Sjögren's Syndrome-A Systematic Review.
Jung JY, Kim JW, Kim HA, Suh CH. Jung JY, et al. Int J Mol Sci. 2021 Nov 29;22(23):12903. doi: 10.3390/ijms222312903. Int J Mol Sci. 2021. PMID: 34884709 Free PMC article. Review. - Proteomic diagnosis of Sjögren's syndrome.
Giusti L, Baldini C, Bazzichi L, Bombardieri S, Lucacchini A. Giusti L, et al. Expert Rev Proteomics. 2007 Dec;4(6):757-67. doi: 10.1586/14789450.4.6.757. Expert Rev Proteomics. 2007. PMID: 18067414 Review.
Cited by
- Biological functions and affected signaling pathways by Long Non-Coding RNAs in the immune system.
Ghahramani Almanghadim H, Karimi B, Valizadeh S, Ghaedi K. Ghahramani Almanghadim H, et al. Noncoding RNA Res. 2024 Sep 6;10:70-90. doi: 10.1016/j.ncrna.2024.09.001. eCollection 2025 Feb. Noncoding RNA Res. 2024. PMID: 39315339 Free PMC article. Review. - Recent Advances in Smart Biomaterials for the Detection and Treatment of Autoimmune Diseases.
Shodeinde AB, Murphy AC, Oldenkamp HF, Potdar AS, Ludolph CM, Peppas NA. Shodeinde AB, et al. Adv Funct Mater. 2020 Sep 10;30(37):1909556. doi: 10.1002/adfm.201909556. Epub 2020 Mar 13. Adv Funct Mater. 2020. PMID: 33071713 Free PMC article. - Non-coding RNAs in saliva: emerging biomarkers for molecular diagnostics.
Majem B, Rigau M, Reventós J, Wong DT. Majem B, et al. Int J Mol Sci. 2015 Apr 17;16(4):8676-98. doi: 10.3390/ijms16048676. Int J Mol Sci. 2015. PMID: 25898412 Free PMC article. Review. - Applications of Mass Spectrometry in Dentistry.
Kallianta M, Pappa E, Vastardis H, Rahiotis C. Kallianta M, et al. Biomedicines. 2023 Jan 19;11(2):286. doi: 10.3390/biomedicines11020286. Biomedicines. 2023. PMID: 36830822 Free PMC article. Review. - Peripheral blood gene expression profiling in Sjögren's syndrome.
Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, Segal B, Rhodus NL, Moser KL. Emamian ES, et al. Genes Immun. 2009 Jun;10(4):285-96. doi: 10.1038/gene.2009.20. Epub 2009 Apr 30. Genes Immun. 2009. PMID: 19404300 Free PMC article.
References
- Sjogren H. Zur Kenntnis der Keratoconjunctivitis sicca. Acta Ophthalmol. 1933;11 Suppl 2:1–151. - PubMed
- Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. and the European Study Group on Classification Criteria for Sjögren’s Syndrome Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. - PMC - PubMed
- Fox R. Sjögren’s syndrome. Lancet. 2005;366:321–331. - PubMed
- Pijpe J, Kalk WW, van der Wal JE, Vissink A, Kluin PM, Roodenburg JL, et al. Parotid gland biopsy compared with labial biopsy in the diagnosis of patients with primary Sjögren’s syndrome. Rheumatology (Oxford) 2007;46:335–341. - PubMed
- Malamud D. Oral diagnostic testing for detecting human immunodeficiency virus-1 antibodies: a technology whose time has come. Am J Med. 1997;102:9–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DE016275-02/DE/NIDCR NIH HHS/United States
- U01-DE-16275/DE/NIDCR NIH HHS/United States
- R01 DE017593-01/DE/NIDCR NIH HHS/United States
- R01-DE-17593/DE/NIDCR NIH HHS/United States
- U01 DE016275/DE/NIDCR NIH HHS/United States
- R01 DE017593/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases