Prognostic value of posteromedial cortex deactivation in mild cognitive impairment - PubMed (original) (raw)
Prognostic value of posteromedial cortex deactivation in mild cognitive impairment
Jeffrey R Petrella et al. PLoS One. 2007.
Abstract
Background: Normal subjects deactivate specific brain regions, notably the posteromedial cortex (PMC), during many tasks. Recent cross-sectional functional magnetic resonance imaging (fMRI) data suggests that deactivation during memory tasks is impaired in Alzheimer's disease (AD). The goal of this study was to prospectively determine the prognostic significance of PMC deactivation in mild cognitive impairment (MCI).
Methodology/principal findings: 75 subjects (34 MCI, 13 AD subjects and 28 controls) underwent baseline fMRI scanning during encoding of novel and familiar face-name pairs. MCI subjects were followed longitudinally to determine conversion to AD. Regression and analysis of covariance models were used to assess the effect of PMC activation/deactivation on conversion to dementia as well as in the longitudinal change in dementia measures. At longitudinal follow up of up to 3.5 years (mean 2.5+/-0.79 years), 11 MCI subjects converted to AD. The proportion of deactivators was significantly different across all groups: controls (79%), MCI-Nonconverters (73%), MCI-converters (45%), and AD (23%) (p<0.05). Mean PMC activation magnitude parameter estimates, at baseline, were negative in the control (-0.57+/-0.12) and MCI-Nonconverter (-0.33+/-0.14) groups, and positive in the MCI-Converter (0.37+/-0.40) and AD (0.92+/-0.30) groups. The effect of diagnosis on PMC deactivation remained significant after adjusting for age, education and baseline Mini-Mental State Exam (p<0.05). Baseline PMC activation magnitude was correlated with change in dementia ratings from baseline.
Conclusion: Loss of physiological functional deactivation in the PMC may have prognostic value in preclinical AD, and could aid in profiling subgroups of MCI subjects at greatest risk for progressive cognitive decline.
Conflict of interest statement
Competing Interests: Dr. Doraiswamy has received research grant support and/or honoraria for consulting or speaking from several pharmaceutical or diagnostic companies and owns stock in Sonexa Therapeutics. Duke University and Dr. Doraiswamy hold a use patent for an unrelated treatment indication in children; that patent is unlicensed and he derives no income from it. Dr. Petrella has received research support from Eisai/Pfizer and AVID for other pilot studies. Dr. Wang and Ms. Hellegers have received salary support from several pharmaceutical companies for other studies. Dr. Prince has no conflicts to disclose.
Figures
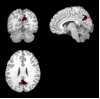
Figure 1. Posteromedical cortex (PMC), denoted in red and overlaid on a canonical T1-weighted brain template image in three orthogonal views, was used as an apriori functional region of interest.
In this region parameter estimates for activation magnitude showed a lesser-to-greater activation from Control, to MCI, to AD subjects.
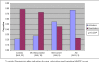
Figure 2. Bar graph demonstrates the proportion of activators and deactivators among the four diagnostic groups.
The proportion of deactivators was significantly different (p<0.05) across diagnostic groups after adjusting for age, education and baseline MMSE score, with significantly fewer deactivators with increasing cognitive impairment grouping.
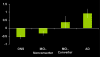
Figure 3. Bar graph demonstrates activation magnitude parameter estimate in the PMC region (taken from Figure 1), demonstrating a continuum from control, to MCI-Nonconverter, to MCI-Converter, to AD.
There were statistically significant (p<0.05) differences between all groups with the exception of the control and MCI-Nonconverter group, and the AD and MCI-Converter group. Note the overall pattern of negative activation magnitude in the Control and MCI-Nonconverter groups, and positive activation magnitude in the AD and MCI-Converter groups.
Figure 4. Scatterplots demonstrate activation magnitude parameter estimate in the PMC region (on the y-axis) significantly (p<0.05) correlated with longitudinal change in cognitive measures (increase in CDR-SOB, decrease in MMSE) in the MCI group.
Similar articles
- Cortical responses to a graded working memory challenge predict functional decline in mild cognitive impairment.
Kochan NA, Breakspear M, Valenzuela M, Slavin MJ, Brodaty H, Wen W, Trollor JN, Turner A, Crawford JD, Sachdev PS. Kochan NA, et al. Biol Psychiatry. 2011 Jul 15;70(2):123-30. doi: 10.1016/j.biopsych.2011.03.006. Epub 2011 May 5. Biol Psychiatry. 2011. PMID: 21546002 - Default mode network connectivity in stable vs progressive mild cognitive impairment.
Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM. Petrella JR, et al. Neurology. 2011 Feb 8;76(6):511-7. doi: 10.1212/WNL.0b013e31820af94e. Epub 2011 Jan 12. Neurology. 2011. PMID: 21228297 Free PMC article. - White matter signal abnormality quality differentiates mild cognitive impairment that converts to Alzheimer's disease from nonconverters.
Lindemer ER, Salat DH, Smith EE, Nguyen K, Fischl B, Greve DN; Alzheimer's Disease Neuroimaging Initiative. Lindemer ER, et al. Neurobiol Aging. 2015 Sep;36(9):2447-57. doi: 10.1016/j.neurobiolaging.2015.05.011. Epub 2015 May 28. Neurobiol Aging. 2015. PMID: 26095760 Free PMC article. - Structural and functional MRI in mild cognitive impairment.
Pihlajamäki M, Jauhiainen AM, Soininen H. Pihlajamäki M, et al. Curr Alzheimer Res. 2009 Apr;6(2):179-85. doi: 10.2174/156720509787602898. Curr Alzheimer Res. 2009. PMID: 19355853 Review. - Neuropsychology of posteromedial parietal cortex and conversion factors from Mild Cognitive Impairment to Alzheimer's disease: systematic search and state-of-the-art review.
Ilardi CR, Chieffi S, Iachini T, Iavarone A. Ilardi CR, et al. Aging Clin Exp Res. 2022 Feb;34(2):289-307. doi: 10.1007/s40520-021-01930-y. Epub 2021 Jul 7. Aging Clin Exp Res. 2022. PMID: 34232485 Free PMC article. Review.
Cited by
- Functional and structural MR imaging in neuropsychiatric disorders, Part 1: imaging techniques and their application in mild cognitive impairment and Alzheimer disease.
Mueller S, Keeser D, Reiser MF, Teipel S, Meindl T. Mueller S, et al. AJNR Am J Neuroradiol. 2012 Nov;33(10):1845-50. doi: 10.3174/ajnr.A2799. Epub 2011 Dec 15. AJNR Am J Neuroradiol. 2012. PMID: 22173754 Free PMC article. Review. - Synaptic depression and aberrant excitatory network activity in Alzheimer's disease: two faces of the same coin?
Palop JJ, Mucke L. Palop JJ, et al. Neuromolecular Med. 2010 Mar;12(1):48-55. doi: 10.1007/s12017-009-8097-7. Epub 2009 Oct 17. Neuromolecular Med. 2010. PMID: 19838821 Free PMC article. Review. - Task-enhanced arterial spin labeled perfusion MRI predicts longitudinal neurodegeneration in mild cognitive impairment.
Xie L, Das SR, Pilania A, Daffner M, Stockbower GE, Dolui S, Yushkevich PA, Detre JA, Wolk DA. Xie L, et al. Hippocampus. 2019 Jan;29(1):26-36. doi: 10.1002/hipo.23026. Epub 2018 Nov 6. Hippocampus. 2019. PMID: 30207006 Free PMC article. - Age and amyloid-related alterations in default network habituation to stimulus repetition.
Vannini P, Hedden T, Becker JA, Sullivan C, Putcha D, Rentz D, Johnson KA, Sperling RA. Vannini P, et al. Neurobiol Aging. 2012 Jul;33(7):1237-52. doi: 10.1016/j.neurobiolaging.2011.01.003. Epub 2011 Feb 18. Neurobiol Aging. 2012. PMID: 21334099 Free PMC article. Clinical Trial. - Potential of functional MRI as a biomarker in early Alzheimer's disease.
Sperling R. Sperling R. Neurobiol Aging. 2011 Dec;32 Suppl 1(Suppl 1):S37-43. doi: 10.1016/j.neurobiolaging.2011.09.009. Neurobiol Aging. 2011. PMID: 22078171 Free PMC article. Review.
References
- Tanzi RE. The synaptic Abeta hypothesis of Alzheimer disease.[comment]. Nature Neuroscience. 2005;8:977–979. - PubMed
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. - PubMed
- Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources