Role of autophagy in cancer - PubMed (original) (raw)
Review
Role of autophagy in cancer
Robin Mathew et al. Nat Rev Cancer. 2007 Dec.
Abstract
Autophagy is a cellular degradation pathway for the clearance of damaged or superfluous proteins and organelles. The recycling of these intracellular constituents also serves as an alternative energy source during periods of metabolic stress to maintain homeostasis and viability. In tumour cells with defects in apoptosis, autophagy allows prolonged survival. Paradoxically, autophagy defects are associated with increased tumorigenesis, but the mechanism behind this has not been determined. Recent evidence suggests that autophagy provides a protective function to limit tumour necrosis and inflammation, and to mitigate genome damage in tumour cells in response to metabolic stress.
Figures
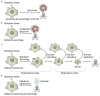
Figure 1. Altered capacity for apoptosis and autophagy can dictate cell fate in response to metabolic stress
a | Apoptosis is a common response to metabolic stress in which cells activate caspases and die efficiently. For immortal epithelial cells, metabolic stress triggers apoptosis within 24 to 48 hours,,. Execution of apoptosis occurs in less than an hour and cell viability loss is five to six orders of magnitude. b | Defective autophagy (through loss of BECN1 or ATG5, for example) can increase apoptotic cell death in some cells in response to metabolic stress. In human mammary cells in 3D culture in vitro, this accelerated apoptosis manifests as increased lumen formation in mammary acini . Therefore, preservation of cell metabolism through autophagy might increase the threshold for apoptosis activation. c | In cells with defects in apoptosis, survival in metabolic stress is dependent on autophagy and is prolonged for weeks,,,. During the maintenance phase, activities such as cell division and motility are sustained. Prolonged starvation and progressive autophagy causes cells to gradually shrink in size but restoration of nutrients allows recovery. In the preservation phase, cell division and motility decrease, presumably as a bioenergenic conservation effort, creating the minimal cell that is capable of recovery (MCCR). Eventually, restoration of nutrients fails to allow recovery. In this way, autophagy can be viewed as an interruptible path to cell death. d | Cells with defects in apoptosis and autophagy fail to tolerate metabolic stress, undergo metabolic catastrophe and die by necrosis.
Figure 2. Role of apoptosis and autophagy in tumorigenesis
a | Tumour-initiating mutational events such as oncogene activation promote cell proliferation, but also apoptosis, which limits tumour growth. Following the acquisition of defects in apoptosis, tumour proliferation is sustained in the absence of apoptotic cell death. b | Tumour growth is initially limited by the absence of a blood supply, which can trigger autophagy-mediated survival in the most metabolically stressed tumour regions, commonly the hypoxic center,,. The eventual recruitment of a blood supply cures the tumour of hypoxia and metabolic stress, and the tumour cells formerly surviving through autophagy can emerge to contribute to tumour growth. c | In tumours formed by cells with defects in both apoptosis and autophagy, necrotic cell death is stimulated in metabolically stressed tumour regions and this necrosis is associated with the activation of an inflammatory response, DNA damage and tumour progression,,. Analogous to a wound-healing response, chronic necrosis and inflammation can stimulate angiogenesis and tumour growth–.
Figure 3. Application of autophagy modulation to cancer therapy
a | In apoptosis-defective tumours that are reliant on autophagy to survive metabolic stress, autophagy inhibitors can be used to induce acute necrotic cell death that may be facilitated by proteasome inhibition, enabling tumour eradication. b | In the adjuvant setting, and after elimination of a large proportion of the tumour by radiation and chemotherapy, the remaining cells can reside in a disrupted and stressed environment, susceptible to inhibition of the autophagy survival mechanism. Tumour cells in the process of metastasis can be similarly vulnerable. c | Autophagy stimulators may be therapeutically useful to either promote autophagic cell death or to prevent the damaging effects of autophagy deficiency and mismanagement of metabolic stress leading to DNA damage and tumour progression. By limiting protein, organelle and ultimately DNA damage, autophagy stimulators may suppress tumour progression. In human breast, ovarian and prostate cancers, where allelic loss of BECN1 occurs with high frequency, correction of the autophagy deficiency with autophagy stimulators may delay tumour progression by reducing the rate at which tumour-promoting mutations accumulate.
Similar articles
- The double-edged sword of autophagy modulation in cancer.
White E, DiPaola RS. White E, et al. Clin Cancer Res. 2009 Sep 1;15(17):5308-16. doi: 10.1158/1078-0432.CCR-07-5023. Epub 2009 Aug 25. Clin Cancer Res. 2009. PMID: 19706824 Free PMC article. Review. - Role of autophagy in suppression of inflammation and cancer.
White E, Karp C, Strohecker AM, Guo Y, Mathew R. White E, et al. Curr Opin Cell Biol. 2010 Apr;22(2):212-7. doi: 10.1016/j.ceb.2009.12.008. Epub 2010 Jan 6. Curr Opin Cell Biol. 2010. PMID: 20056400 Free PMC article. Review. - p53 and autophagy in cancer: guardian of the genome meets guardian of the proteome.
Ryan KM. Ryan KM. Eur J Cancer. 2011 Jan;47(1):44-50. doi: 10.1016/j.ejca.2010.10.020. Epub 2010 Nov 25. Eur J Cancer. 2011. PMID: 21112207 Review. - Autophagy in tumour suppression and promotion.
Brech A, Ahlquist T, Lothe RA, Stenmark H. Brech A, et al. Mol Oncol. 2009 Aug;3(4):366-75. doi: 10.1016/j.molonc.2009.05.007. Epub 2009 Jun 6. Mol Oncol. 2009. PMID: 19559660 Free PMC article. Review. - Cancer-type-specific crosstalk between autophagy, necroptosis and apoptosis as a pharmacological target.
Radogna F, Dicato M, Diederich M. Radogna F, et al. Biochem Pharmacol. 2015 Mar 1;94(1):1-11. doi: 10.1016/j.bcp.2014.12.018. Epub 2015 Jan 3. Biochem Pharmacol. 2015. PMID: 25562745 Review.
Cited by
- Nucleocytoplasmic transport blockage by SV40 peptide-modified gold nanoparticles induces cellular autophagy.
Tsai TL, Hou CC, Wang HC, Yang ZS, Yeh CS, Shieh DB, Su WC. Tsai TL, et al. Int J Nanomedicine. 2012;7:5215-34. doi: 10.2147/IJN.S35125. Epub 2012 Oct 8. Int J Nanomedicine. 2012. PMID: 23071392 Free PMC article. - The Role of Autophagy in Salivary Gland Homeostasis and Stress Responses.
Morgan-Bathke M, Lin HH, Ann DK, Limesand KH. Morgan-Bathke M, et al. J Dent Res. 2015 Aug;94(8):1035-40. doi: 10.1177/0022034515590796. Epub 2015 Jun 19. J Dent Res. 2015. PMID: 26092378 Free PMC article. Review. - The complexity of p53-mediated metabolic regulation in tumor suppression.
Liu Y, Gu W. Liu Y, et al. Semin Cancer Biol. 2022 Oct;85:4-32. doi: 10.1016/j.semcancer.2021.03.010. Epub 2021 Mar 27. Semin Cancer Biol. 2022. PMID: 33785447 Free PMC article. Review. - MiR-221/222 promote migration and invasion, and inhibit autophagy and apoptosis by modulating ATG10 in aggressive papillary thyroid carcinoma.
Shen H, Lin Z, Shi H, Wu L, Ma B, Li H, Yin B, Tang J, Yu H, Yin X. Shen H, et al. 3 Biotech. 2020 Aug;10(8):339. doi: 10.1007/s13205-020-02326-x. Epub 2020 Jul 15. 3 Biotech. 2020. PMID: 32704465 Free PMC article. - Selective and potent proteomimetic inhibitors of intracellular protein-protein interactions.
Barnard A, Long K, Martin HL, Miles JA, Edwards TA, Tomlinson DC, Macdonald A, Wilson AJ. Barnard A, et al. Angew Chem Int Ed Engl. 2015 Mar 2;54(10):2960-5. doi: 10.1002/anie.201410810. Epub 2015 Feb 4. Angew Chem Int Ed Engl. 2015. PMID: 25651514 Free PMC article.
References
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. - PubMed
- Mizushima N. Autophagy: process and function. Genes Dev. (in the press) - PubMed
- Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. - PubMed
- Komatsu M, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- L30 CA116898/CA/NCI NIH HHS/United States
- L30 CA116898-02/CA/NCI NIH HHS/United States
- L30 CA116898-01/CA/NCI NIH HHS/United States
- R37 CA053370-17/CA/NCI NIH HHS/United States
- R37 CA053370/CA/NCI NIH HHS/United States
- R37 CA053370-16/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources