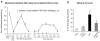p38 MAPK, microglial signaling, and neuropathic pain - PubMed (original) (raw)
Review
p38 MAPK, microglial signaling, and neuropathic pain
Ru-Rong Ji et al. Mol Pain. 2007.
Abstract
Accumulating evidence over last several years indicates an important role of microglial cells in the pathogenesis of neuropathic pain. Signal transduction in microglia under chronic pain states has begun to be revealed. We will review the evidence that p38 MAPK is activated in spinal microglia after nerve injury and contributes importantly to neuropathic pain development and maintenance. We will discuss the upstream mechanisms causing p38 activation in spinal microglia after nerve injury. We will also discuss the downstream mechanisms by which p38 produces inflammatory mediators. Taken together, current data suggest that p38 plays a critical role in microglial signaling under neuropathic pain conditions and represents a valuable therapeutic target for neuropathic pain management.
Figures
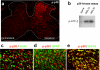
Figure 1
Ligation of L5-spinal nerve (SNL) induces p38 activation in microglial cells in the spinal cord. (a) Immunostaining of phosphorylated p38 (p-p38) in the spinal cord 3 days after SNL. Dotted line indicates the outline of the spinal cord gray matter. Modified from [34]. (b) p38 kinase assay reveals that SNL increases the phosphorylation of the p38 substrate ATF-2, suggesting an increase in p38 activity. To perform kinase assay, p-p38 was first immunoprecipitated with a p-p38 antibody, then ATF-2 fusion protein was added. Finally, pATF-2 level was detected using Western blotting with a pATF-2 antibody. (c-e) Double immunofluorescence indicates that p-p38 is not colocalized with NeuN, a neuronal marker (c) and GFAP, an astroglial marker (d), but colocalized with OX-42 (CD11b), a microglial marker (e). Modified from [34].
Figure 2
(a) Reversal of tactile allodynia by p38 inhibition after spinal nerve ligation (SNL) on mice. The p38 inhibitor FR167653 was injected (i.p., 50 mg/kg, in 10% DMSO) at 10 days after SNL. The same injection was repeated daily for additional 4 days (indicated with small arrows). SNL-induced mechanical allodynia was tested at 1 h, 3 h, 6 h, and 1 d after the first injection, then daily for another 6 days. Mean ± SEM (n = 5). Note that p38 inhibitor is still effective in reversing neuropathic pain. Interestingly, the effect is maintained for 2 more days after the last injection. (b) Inhibition of SNL-induced IL-1β upregulation in the spinal cord by p38 inhibition. ELISA shows that intrathecal injection of the p38 inhibitor SB203580 (10 μg, twice a day for 3 days) reduces SNL-induced IL-1β increase in the L5 spinal cord (dorsal part) 3 days after SNL (L5). **, P < 0.01, compared to Sham; +, P < 0.05, compared to SNL, ANOVA.
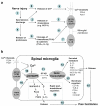
Figure 3
Schematic representation of signal transduction in spinal microglia after nerve injury. (a) Activation of microglial receptors after nerve injury. Peripheral nerve injury generates spontaneous activity (step-1) leading to the release of chemokines (e.g. MCP-1) from primary sensory DRG neurons (step-2). MCP-1 will activate CCR2 receptors on microglia (step-3). Spontaneous activity may also release the proteinases, leading to the cleavage of the chemokine FKN (step-5). After its cleavage from the membrane, FKN will be released to bind CX3CR1 receptor on microglia (step-6). Proteinases may also cleave the precursors of the cytokines TNFα and IL-1β, leading to the activation of TNFα and IL-1β receptors on microglia (step-7). Nerve injury will further release ATP (step-8), activating P2X4 and P2X7 receptors on microglia (step-9). (b) p38 activation in spinal microglia and downstream signaling of p38. Activation of GPCR, or cytokine receptors, or P2X receptors results in p38 MAPK activation in spinal microglia (step-10). p38 activation results in increased expression, through the transcription factor NF-κB (step-11) or other transcription factors (e.g. ATF-2), of secreted inflammatory mediators/growth factors (e.g., cytokines and BDNF, step-12) or of genes encoding membrane receptors (step-13). In addition, p38 also induces release of PGE2 and IL-1β via rapid posttranslational regulation (step-14). Upon release, these mediators will sensitize nociceptive dorsal horn neurons via presynaptic and postsynaptic mechanisms, leading to persistent pain hypersensitivity (step-15). Abbreviations used in Fig 3: BDNF, brain-derived neurotrophic factor; CatS, cysteine protease cathepsin S; DRG, dorsal root ganglion; FKN, fractalkine; GPCR, G-protein coupled receptor; IL-1β, interleukin-1beta; MAPK, mitogen-activated protein kinase; MAPKAP2: MAPK-activated protein kinase-2; PLA2, phospholipase A2; TNFα, tumor necrosis factor-alpha
Similar articles
- Interleukin-6 induces microglial CX3CR1 expression in the spinal cord after peripheral nerve injury through the activation of p38 MAPK.
Lee KM, Jeon SM, Cho HJ. Lee KM, et al. Eur J Pain. 2010 Aug;14(7):682.e1-12. doi: 10.1016/j.ejpain.2009.10.017. Epub 2009 Dec 2. Eur J Pain. 2010. PMID: 19959384 - Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine.
Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Zhuang ZY, et al. Brain Behav Immun. 2007 Jul;21(5):642-51. doi: 10.1016/j.bbi.2006.11.003. Epub 2006 Dec 15. Brain Behav Immun. 2007. PMID: 17174525 Free PMC article. - Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord.
Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, Ji RR. Taves S, et al. Brain Behav Immun. 2016 Jul;55:70-81. doi: 10.1016/j.bbi.2015.10.006. Epub 2015 Oct 19. Brain Behav Immun. 2016. PMID: 26472019 Free PMC article. - Microglia: a promising target for treating neuropathic and postoperative pain, and morphine tolerance.
Wen YR, Tan PH, Cheng JK, Liu YC, Ji RR. Wen YR, et al. J Formos Med Assoc. 2011 Aug;110(8):487-94. doi: 10.1016/S0929-6646(11)60074-0. J Formos Med Assoc. 2011. PMID: 21783017 Free PMC article. Review. - Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia.
Tsuda M, Inoue K, Salter MW. Tsuda M, et al. Trends Neurosci. 2005 Feb;28(2):101-7. doi: 10.1016/j.tins.2004.12.002. Trends Neurosci. 2005. PMID: 15667933 Review.
Cited by
- Activation of different signals identified with glia cells contribute to the progression of hyperalgesia.
Yamamoto S, Kishishita Y, Yoshida M, Miura D, Suzuki H, Ishikawa K, Miyazaki H, Nojima J, Yamamoto M, Ishikawa T. Yamamoto S, et al. Cell Mol Neurobiol. 2013 Mar;33(2):167-74. doi: 10.1007/s10571-012-9881-8. Epub 2012 Oct 23. Cell Mol Neurobiol. 2013. PMID: 23208699 Free PMC article. - Altered spinal microRNA-146a and the microRNA-183 cluster contribute to osteoarthritic pain in knee joints.
Li X, Kroin JS, Kc R, Gibson G, Chen D, Corbett GT, Pahan K, Fayyaz S, Kim JS, van Wijnen AJ, Suh J, Kim SG, Im HJ. Li X, et al. J Bone Miner Res. 2013 Dec;28(12):2512-22. doi: 10.1002/jbmr.2002. J Bone Miner Res. 2013. PMID: 23744481 Free PMC article. - TRP Channels Role in Pain Associated With Neurodegenerative Diseases.
Duitama M, Vargas-López V, Casas Z, Albarracin SL, Sutachan JJ, Torres YP. Duitama M, et al. Front Neurosci. 2020 Aug 4;14:782. doi: 10.3389/fnins.2020.00782. eCollection 2020. Front Neurosci. 2020. PMID: 32848557 Free PMC article. Review. - Systemic minocycline differentially influences changes in spinal microglial markers following formalin-induced nociception.
Li K, Fu KY, Light AR, Mao J. Li K, et al. J Neuroimmunol. 2010 Apr 15;221(1-2):25-31. doi: 10.1016/j.jneuroim.2010.02.003. Epub 2010 Mar 4. J Neuroimmunol. 2010. PMID: 20202692 Free PMC article. - P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide.
Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M. Clark AK, et al. J Neurosci. 2010 Jan 13;30(2):573-82. doi: 10.1523/JNEUROSCI.3295-09.2010. J Neurosci. 2010. PMID: 20071520 Free PMC article.
References
- Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;2004:reE14. - PubMed
- Devor M, Seltzer Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD and Melzack R, editor. Textbook of Pain. 4th. Vol. 5. Edinburgh, Churchill Livingstone; 1999. pp. 129–164.
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS054932/NS/NINDS NIH HHS/United States
- NS40698/NS/NINDS NIH HHS/United States
- TW7180/TW/FIC NIH HHS/United States
- R01 DE017794/DE/NIDCR NIH HHS/United States
- R03 TW007180/TW/FIC NIH HHS/United States
- DE17794/DE/NIDCR NIH HHS/United States
- R01 NS040698/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources