Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia - PubMed (original) (raw)
Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia
Hua Zhang et al. J Neurosci. 2007.
Abstract
The neuropeptide substance P (SP) is expressed in unmyelinated primary sensory neurons and represents the best known "pain" neurotransmitter. It is generally believed that SP regulates pain transmission and sensitization by acting on neurokinin-1 receptor (NK-1), which is expressed in postsynaptic dorsal horn neurons. However, the expression and role of NK-1 in primary sensory neurons are not clearly characterized. Our data showed that NK-1 was expressed in both intact and dissociated dorsal root ganglion (DRG) neurons. In particular, NK-1 was mainly coexpressed with the capsaicin receptor TRPV1 (transient receptor potential vanilloid subtype 1), a critical receptor for the generation of heat hyperalgesia. NK-1 agonist [Sar(9), Met(O2)(11)]-substance P (Sar-SP) significantly potentiated capsaicin-induced currents and increase of [Ca2+]i in dissociated DRG neurons. NK-1 antagonist blocked not only the potentiation of TRPV1 currents but also heat hyperalgesia induced by intraplantar Sar-SP. NK-1 antagonist also inhibited capsaicin-induced spontaneous pain, and this inhibition was enhanced after inflammation. To analyze intracellular cross talking of NK-1 and TRPV1, we examined downstream signal pathways of G-protein-coupled NK-1 activation. Sar-SP-induced potentiation of TRPV1 was blocked by inhibition of G-protein, PLCbeta (phospholipase C-beta), or PKC but not by inhibition of PKA (protein kinase A). In particular, PKCepsilon inhibitor completely blocked both Sar-SP-induced TRPV1 potentiation and heat hyperalgesia. Sar-SP also induced membrane translocation of PKCepsilon in a portion of small DRG neurons. These results reveal a novel mechanism of NK-1 in primary sensory neurons via a possible autocrine and paracrine action of SP. Activation of NK-1 in these neurons induces heat hyperalgesia via PKCepsilon-mediated potentiation of TRPV1.
Figures
Figure 1.
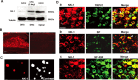
Expression of NK-1 in rat DRG neurons. A, Western blotting shows NK-1 expression in the DRG and spinal dorsal horn. Note that inflammation by CFA injection increases NK-1 expression. β-Tubulin serves as the loading control. This result has been repeated in three rats. B, Immunofluorescence of NK-1 in the DRG section. Left, NK-1 expression in many DRG neurons. Right, Absence of NK-1 staining after omission of NK-1 primary antibody. C, Immunofluorescence of NK-1 in acutely isolated DRG neurons. Left, NK-1 expression in small DRG neurons. Right, A contrast image showing all neurons in the same field shown in the left panel. D, Colocalization of NK-1 with TRPV1 (a), SP (b), and NF-200 (c) in DRG neurons of naive rats. Two single-stained images were merged (right) to demonstrate double staining. Arrowheads indicate double staining in the cytoplasm. Small arrows indicate double staining on the membrane. It is notable that NK-1 is only expressed on the membrane of some small DRG neurons. Scale bars: B, D, 50 μm; C, 25 μm.
Figure 2.
SP and the NK-1 agonist Sar-SP potentiate TRPV1 function in small DRG neurons. A, Representative trace of SP-induced potentiation of capsaicin (500 n
m
, 5 s)-activated current in rat DRG neurons. Cells were perfused for 3 min with SP (1 μ
m
) before the second capsaicin application. The inset shows the fold change of capsaicin currents in sensitized and nonsensitized cells. B, NK-1 agonist Sar-SP (1 μ
m
) enhanced capsaicin (500 n
m
, 5 s)-activated current in a time-dependent manner. The inset indicates the time course of the potentiating effect after Sar-SP treatment. *p < 0.05, ***p < 0.001 versus pre-Sar-SP treatment (unpaired t test). C, Effects of SP, Sar-SP, and SP fragments (SP1–7, SP5–11), all at the concentration of 1 μ
m
, on capsaicin currents. SP-induced potentiation of capsaicin currents is blocked by the NK-1 antagonist (GR82334, 1 μ
m
) but not by the NK-2/3 antagonists (L659,877 and SR-142,801, 1 μ
m
). ***p < 0.001 versus pre-SP treatment. D, Sar-SP (1 μ
m
) enhanced capsaicin-induced increases in [Ca2+]i in a time-dependent manner. E, Dose-dependent effects of Sar-SP potentiation of capsaicin currents. The maximum currents were measured after a 1 min application of different concentrations of Sar-SP. EC50 = 0.522 ± 0.133 μ
m
(n = 7). F, Summary of Sar-SP effect of on higher concentrations of capsaicin-induced currents with and without extracellular Ca2+. Cap, Capsaicin. *p < 0.05; **p < 0.01.
Figure 3.
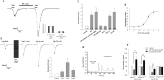
Sar-SP enhances sensitivity of DRG neurons in response to thermal stimulation. A, Sar-SP potentiates the heat-evoked currents. Perfusion of a 30 s preheated solution enhanced the temperature around the cell to the peak of 45°C. Sar-SP both enhanced the amplitude of the heat-evoked current and reduced the threshold of current activation (from 41.7 to 38.4°C). Normal solution temperature is the temperature before heat stimulation. The inset shows capsaicin (Cap) response of the recorded cell tested at the end of the experiment. B, C, Comparison of amplitude and threshold change of heat-evoked currents before and after application of Sar-SP. *p < 0.001 versus the threshold before Sar-SP application. D, Effects of SP on the thermal threshold of heat-evoked current. After application of preheated solution for 20 s, the temperature around the recorded cells increased to a level that is just below normal thermal threshold (42°C). In the absence of Sar-SP, this subthreshold temperature did not induce any currents in all 10 recorded cells. After application of Sar-SP, the same stimulation protocol induced inward currents in five cells, and the thermal threshold was reduced to 36.5 ± 1.5°C. The inset shows capsaicin response of the recorded cell tested at the end of the experiment. T, Temperature.
Figure 4.
TRPV1 potentiation by NK-1 activation is mediated by G-protein and PKC. A, Intracellular application of GDP-β-S (1 m
m
), a G-protein inhibitor, abolished Sar-SP-induced enhancement of TRPV1 currents. The inset shows fold change of the currents. ***p < 0.001 (vs control group) (n = 18). B, PKC inhibitor BIM (1 μ
m
) prevented Sar-SP (1 μ
m
)-induced TRPV1 current enhancement. Note that BIM alone had no effect on TRPV1 current, and after washout, Sar-SP was able to potentiate the current again. The inset is the statistic result (n = 18). ***p < 0.001 versus control group, C, Pretreatment of DRG neurons with BIM also abolished Sar-SP-induced potentiation of capsaicin response indicated by calcium imaging, another way of showing TRPV1 sensitization. ***p < 0.001 versus Sar-SP plus capsaicin. The values inside or under the columns indicate the numbers of responders to NK-1 versus the total number of TRPV1-positive neurons tested. When the antagonists were tested, the data show all the neurons tested that had baseline capsaicin responses. D, After TRPV1 sensitization by the PKC activator PMA (0.3 μ
m
, 1 min), Sar-SP failed to further enhance the TRPV1 sensitivity. Cap, Capsaicin.
Figure 5.
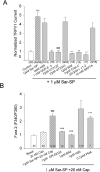
A, Summary histogram shows the effects of different reagents on Sar-SP potentiation of capsaicin (500 n
m
) currents. Currents are expressed as fold of the values of basal capsaicin currents. Sar-SP evoked a strong potentiation of capsaicin currents in 34 of 59 cells (+++p < 0.001 vs the control TRPV1 currents). Intracellular application of GDP-β-S (1 m
m
) or εV1-2 (200 μ
m
), or bath application of BIM, Chel.C, and U73122 but not U73343 (inactive control of U73122), inhibited the Sar-SP-evoked potentiation (***p < 0.001 vs the Sar-SP group; ###p < 0.001 vs the U73343 group). Bath application of H89 (1 μ
m
) or eliminating intracellular calcium by BAPTA (10 m
m
) did not prevent the Sar-SP potentiation (p > 0.05). B, Summary of the effects of different reagents on Sar-SP enhancement of capsaicin (Cap)-induced calcium increase. Averaged Fura 2 ratios of positive neurons were analyzed. Similar to the patch-clamp results, Sar-SP significantly increased capsaicin (20 n
m
)-induced the Fura 2 ratio (###p < 0.001). Preincubation of GR82334 or BIM prevented the potentiation of Sar-SP (***p < 0.001 vs the Sar-SP group). Capsaicin-induced calcium increase was also enhanced by the PKC activator PMA (0.3 μ
m
) (+++p < 0.001 vs the 20 n
m
capsaicin-treated group). However, the PKA inhibitor H89 (1 μ
m
) had no effect. The values inside or under the columns indicate the numbers of responders to NK-1 versus the total number of TRPV1-positive neurons tested. When the antagonists were tested, the data show all the neurons tested that had baseline capsaicin responses.
Figure 6.
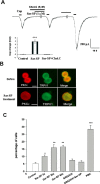
NK-1 activation induces membrane translocation of PKCε in DRG neurons that coexpress TRPV1. A, Involvement of PKC translocation in Sar-SP induced TRPV1 current enhancement. Coapplication of PKC translocation inhibitor Chel.C (5 μ
m
) and Sar-SP (1 μ
m
) failed to enhance the TRPV1 current. After washout, Sar-SP was still able to enhance the TRPV1 current. The inset summarizes the effect of Chel.C (***p < 0.001 vs control group; n = 15). Cap, Capsaicin. B, Double staining of PKCε (red) and TRPV1 (green) in dissociated DRG neurons before and after (1 min) Sar-SP (1 μ
m
) treatment. PKCε and TRPV1 were heavily colocalized in small DRG neurons. Before Sar-SP (1 μ
m
) treatment, PKCε was found in the cytoplasm, whereas TRPV1 was present both in the cytoplasm and on the membrane (top). After Sar-SP application, PKCε was greatly translocated to the plasma membrane. Scale bars, 20 μm. C, Percentage of neurons demonstrating PKCε membrane translocation after Sar-SP (1 μ
m
for 5, 30, or 60 s). Pretreatment with the NK-1-specific antagonist GR82334 (1 μ
m
, 15 min) completely prevented Sar-SP (1 min)-induced PKCε translocation. The PKC activator PMA serves as a positive control for the translocation (*p < 0.05, **p < 0.01, ***p < 0.001 vs the control group; n = 3).
Figure 7.
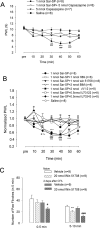
Involvement of peripheral NK-1 activation in thermal hyperalgesia. A, Intraplantar SP-induced thermal hyperalgesia is mediated by TRPV1. Heat hyperalgesia, which was determined by PWL, was induced after intraplantar injection of Sar-SP but not saline. Pretreatment of capsazepine (5 nmol, intraplantar) completely prevented Sar-SP-induced hyperalgesia (###p < 0.001 compared with the saline group; **p < 0.01, ***p < 0.001 compared with the 1 nmol Sar-SP+5 nmol Capsazepine group). Intraplantar capsazepine (5 nmol) did not affect the basal PWL of normal rats. B, Pretreatment by intraplantar administration of NK-1 inhibitor Win 51708 (1 nmol; filled triangles), PKC inhibitor BIM (2 nmol; open triangle), PKCε inhibitor Myr-εv1–2 (2 nmol; filled squares), or U73122 (0.5 nmol; open squares) but not U73343 (inactive control of U73122, 0.5 nmol; filled diamonds) prevented Sar-SP-induced thermal hyperalgesia. Pretreatment of the PKA inhibitor H89 (1 nmol; open circles) failed to prevent the hyperalgesia (filled circles; n = 8). PWLs are expressed as fold of basal level. *p < 0.05, **p < 0.01, ***p < 0.001, Sar-SP injection versus the saline group at each time point. #p < 0.05, ##p < 0.01, ###p < 0.001, Sar-SP+H89 versus saline at each time point (n = 8). +p < 0.05, ++p < 0.01, +++p < 0.001, Sar-SP+U73343 versus Sar-SP+U73122 at each time point (n = 8). Group difference was compared by ANOVA, followed by a post hoc test. C, Effects of peripheral blockade of NK-1 on capsaicin-induced paw flinching in noninflamed and inflamed rats. Two days after CFA injection into the unilateral handpaw, intraplantar injection of capsaicin (0.1%, 10 μl) induced paw flinching, a spontaneous pain, and this spontaneous pain was watched for 10 min, separated by two phases (0–5 and 5–10 min). NK-1 antagonist Win 51708 (20 nmol, intraplantar) was given 10 min before capsaicin injection. Win 51708 reduced the capsaicin-induced second-phase (5–10 min) paw flinching in both inflamed and normal rats. Win 51708 also reduced the first-phase (0–5 min) paw flinching in the inflamed rats. *p < 0.05 versus vehicle of the normal group; #p < 0.05, ###p < 0.001 versus vehicle of the CFA-inflamed group.
Similar articles
- PKCepsilon-dependent potentiation of TTX-resistant Nav1.8 current by neurokinin-1 receptor activation in rat dorsal root ganglion neurons.
Cang CL, Zhang H, Zhang YQ, Zhao ZQ. Cang CL, et al. Mol Pain. 2009 Jun 30;5:33. doi: 10.1186/1744-8069-5-33. Mol Pain. 2009. PMID: 19563686 Free PMC article. - Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice.
Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Amadesi S, et al. J Physiol. 2006 Sep 1;575(Pt 2):555-71. doi: 10.1113/jphysiol.2006.111534. Epub 2006 Jun 22. J Physiol. 2006. PMID: 16793902 Free PMC article. - Phosphorylation of TRPV1 by neurokinin-1 receptor agonist exaggerates the capsaicin-mediated substance P release from cultured rat dorsal root ganglion neurons.
Tang HB, Li YS, Miyano K, Nakata Y. Tang HB, et al. Neuropharmacology. 2008 Dec;55(8):1405-11. doi: 10.1016/j.neuropharm.2008.08.037. Epub 2008 Sep 17. Neuropharmacology. 2008. PMID: 18809416 - Neurotrophic Factors and Nociceptor Sensitization.
Jankowski MP, Koerber HR. Jankowski MP, et al. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 2. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 2. PMID: 21882462 Free Books & Documents. Review. - The unsilent majority-TRPV1 drives "spontaneous" transmission of unmyelinated primary afferents within cardiorespiratory NTS.
Andresen MC, Hofmann ME, Fawley JA. Andresen MC, et al. Am J Physiol Regul Integr Comp Physiol. 2012 Dec 15;303(12):R1207-16. doi: 10.1152/ajpregu.00398.2012. Epub 2012 Oct 17. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 23076872 Free PMC article. Review.
Cited by
- Molecular and cellular mechanisms that initiate pain and itch.
Luo J, Feng J, Liu S, Walters ET, Hu H. Luo J, et al. Cell Mol Life Sci. 2015 Sep;72(17):3201-23. doi: 10.1007/s00018-015-1904-4. Epub 2015 Apr 18. Cell Mol Life Sci. 2015. PMID: 25894692 Free PMC article. Review. - Distinct modulations of human capsaicin receptor by protons and magnesium through different domains.
Wang S, Poon K, Oswald RE, Chuang HH. Wang S, et al. J Biol Chem. 2010 Apr 9;285(15):11547-56. doi: 10.1074/jbc.M109.058727. Epub 2010 Feb 9. J Biol Chem. 2010. PMID: 20145248 Free PMC article. - PKCepsilon-dependent potentiation of TTX-resistant Nav1.8 current by neurokinin-1 receptor activation in rat dorsal root ganglion neurons.
Cang CL, Zhang H, Zhang YQ, Zhao ZQ. Cang CL, et al. Mol Pain. 2009 Jun 30;5:33. doi: 10.1186/1744-8069-5-33. Mol Pain. 2009. PMID: 19563686 Free PMC article. - Suppression of microRNA-155 attenuates neuropathic pain by regulating SOCS1 signalling pathway.
Tan Y, Yang J, Xiang K, Tan Q, Guo Q. Tan Y, et al. Neurochem Res. 2015 Mar;40(3):550-60. doi: 10.1007/s11064-014-1500-2. Epub 2014 Dec 9. Neurochem Res. 2015. PMID: 25488154 - αCGRP is essential for algesic exocytotic mobilization of TRPV1 channels in peptidergic nociceptors.
Devesa I, Ferrándiz-Huertas C, Mathivanan S, Wolf C, Luján R, Changeux JP, Ferrer-Montiel A. Devesa I, et al. Proc Natl Acad Sci U S A. 2014 Dec 23;111(51):18345-50. doi: 10.1073/pnas.1420252111. Epub 2014 Dec 8. Proc Natl Acad Sci U S A. 2014. PMID: 25489075 Free PMC article.
References
- Akasu T, Ishimatsu M, Yamada K. Tachykinins cause inward current through NK1 receptors in bullfrog sensory neurons. Brain Res. 1996;713:160–167. - PubMed
- Amadesi S, Nie JJ, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis B, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300–4312. - PMC - PubMed
- Andoh T, Nagasawa T, Kuraishi Y. Expression of tachykinin NK1 receptor mRNA in dorsal root ganglia of the mouse. Brain Res Mol Brain Res. 1996;35:329–332. - PubMed
- Bhave G, Zhu WG, Wang HB, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS054932/NS/NINDS NIH HHS/United States
- TW 007180/TW/FIC NIH HHS/United States
- R03 TW007180/TW/FIC NIH HHS/United States
- DE17794/DE/NIDCR NIH HHS/United States
- R01 DE017794/DE/NIDCR NIH HHS/United States
- NS54932/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous