A motif in the clathrin heavy chain required for the Hsc70/auxilin uncoating reaction - PubMed (original) (raw)
A motif in the clathrin heavy chain required for the Hsc70/auxilin uncoating reaction
Iris Rapoport et al. Mol Biol Cell. 2008 Jan.
Abstract
The 70-kDa heat-shock cognate protein (Hsc70) chaperone is an ATP-dependent "disassembly enzyme" for many subcellular structures, including clathrin-coated vesicles where it functions as an uncoating ATPase. Hsc70, and its cochaperone auxilin together catalyze coat disassembly. Like other members of the Hsp70 chaperone family, it is thought that ATP-bound Hsc70 recognizes the clathrin triskelion through an unfolded exposed hydrophobic segment. The best candidate is the unstructured C terminus (residues 1631-1675) of the heavy chain at the foot of the tripod below the hub, containing the sequence motif QLMLT, closely related to the sequence bound preferentially by the substrate groove of Hsc70 (Fotin et al., 2004b). To test this hypothesis, we generated in insect cells recombinant mammalian triskelions that in vitro form clathrin cages and clathrin/AP-2 coats exactly like those assembled from native clathrin. We show that coats assembled from recombinant clathrin are good substrates for ATP- and auxilin-dependent, Hsc70-catalyzed uncoating. Finally, we show that this uncoating reaction proceeds normally when the coats contain recombinant heavy chains truncated C-terminal to the QLMLT motif, but very inefficiently when the motif is absent. Thus, the QLMLT motif is required for Hsc-70-facilitated uncoating, consistent with the proposal that this sequence is a specific target of the chaperone.
Figures
Figure 1.
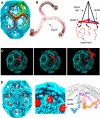
The clathrin triskelion and spatial relationships within a coat. (A) Three-dimensional image reconstruction of a clathrin coat at 8-Å resolution (Fotin et al., 2004b). The colored triskelions show three symmetry-independent molecules. (B) Backbone model of a triskelion for residues 1-1630, highlighting the location of its tripod underneath the hub, and schematic representation of the tripod as it relates to the ankles of legs from adjacent triskelions. (C) Schematic representation of how a relatively rigid rotation of a triskelion about its axis could “unlock” it from the coat, allowing withdrawal from the lattice without colliding with the remaining elements. (D) Three-dimensional image reconstruction of a clathrin coat with bound auxilin (547-910) at 12-Å resolution (Fotin et al., 2004a).
Figure 2.
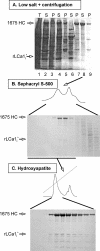
Purification of recombinant clathrin. Analysis by SDS-PAGE and Coomassie Blue staining of fractions obtained during the purification of recombinant clathrin expressed in insect cells. This example shows the results for recombinant full-length rat heavy (1675 HC) and the main proteolytic product of light chain LCa1 (rLCa1i*); details of the purification scheme are given in Materials and Methods. (A) Sample after cell lysis (lane 1); high-speed supernatant (lane 2) and pellet (lane 3) of the cell lysate; low-speed supernatant (lane 4) and pellet (lane 5) of sample in lane 2 after dialysis against low ionic strength solution; high-speed supernatant (lane 6) and pellet (lane 7) of sample in lane 4; high-speed supernatant (lane 8) and pellet (lane 9) of sample in lane 7 after addition of Tris solution (to depolymerize clathrin lattices). The samples correspond to equivalent fractions of the input (T), high-speed supernatant (S) and pellet (P) after centrifugation. (B) Fractionation by gel filtration of sample in lane 8. The figure shows fractions enriched in proteins eluting in the included volume; the elution time and profile of the first peak containing recombinant clathrin is the same as that of native triskelions (data not shown). (C) Elution profile of recombinant clathrin from an hydroxyapatite column loaded with the clathrin pool from B (underlined).
Figure 3.
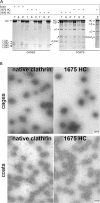
Cage and coat assembly. (A) SDS-PAGE of cages and coats assembled as described in the text. The samples correspond to equivalent fractions of the input (T), high-speed supernatant (S), and pellet (P) after centrifugation of assemblies generated using native or recombinant clathrin (1675 HC and 1630 HC). Coats were assembled in the presence of AP-2. The bands corresponding to clathrin heavy chain (HC), native bovine brain light chains (LCa1, LCa3, LCb2, and LCb3), and recombinant rat light chain (rLCA1i and rLCA1b) are labeled; native bovine AP-2 large-chains (α/β) and AP-2 medium chain (μ2) are also labeled. The ς chain is not visible in the Figure. (B) Electron micrographs of fields of negatively stained clathrin cages or coats made from native or full-length (1675 HC) recombinant clathrin; coats were assembled in the presence of AP-2. The samples are from pellets obtained by high-speed centrifugation after resuspension in cage or coat buffer, respectively. Bar, 100 nm.
Figure 4.
Disassembly of clathrin coats. (A) Clathrin coats assembled in vitro with native clathrin and AP-2 were incubated with Hsc70 and Auxilin for 5 min at 37°C in the absence or presence of ATP, followed by high-speed centrifugation. The protein composition of the supernatants (S) and pellets (P) was analyzed by SDS-PAGE. HC and LCs, bovine brain clathrin heavy and light chains LCa1 and LCb2; α/β and μ2, components of the AP-2 complex. (B) The amino-acid sequence of the C-terminal segment of rat clathrin heavy chain is shown, and the proposed Hsc70 recognition sequence (QLMLT) is highlighted. The schematic representations indicate the truncated heavy chain constructs used in the uncoating experiments. In vitro-assembled coats were subjected to the uncoating reaction, and the extent of uncoating is expressed as percentage of coat proteins remaining in the supernatant of a high-speed centrifugation step as determined by SDS-PAGE analysis. n, number of independent experiments; data are presented as average ± SD. (C) Examples of supernatants analyzed by SDS-PAGE from uncoating reactions after high-speed centrifugation by using as substrate coats assembled with native bovine brain triskelions or with recombinant triskelions made of heavy (1661 HC, 1643 HC, and 1637 HC) and light chains (rLCa1i).
Figure 5.
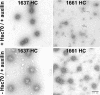
Visualization of coat disassembly. Electron micrographs of negatively stained coats made from recombinant clathrin lacking part (1661 HC) or the complete C-terminal unstructured segment (1637 HC) and AP-2. The images are from pellets resuspended in coat buffer after high-speed centrifugation of samples subjected to the uncoating reaction in the presence of auxilin and ATP and the presence (top) or absence (bottom) of Hsc70. Bar, 100 nm.
Figure 6.
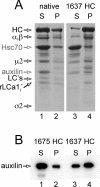
Binding of auxilin to clathrin coats. Coats assembled with native or recombinant full-length (1675 HC) or C-terminally truncated (1637 HC) clathrin were incubated with Hsc70, auxilin, and ATP and subjected to the uncoating reaction (see Materials and Methods). The products of the reaction were pelleted by high-speed centrifugation, and the protein composition of the supernatants and resuspended pellets was analyzed by SDS-PAGE and Coomassie Blue staining (A) or SDS-PAGE and Western blot (B). The auxilin fragment is soluble and occurs mainly in the supernatant fraction (lane 1) of the uncoated native and 1675 HC coats; in contrast, the auxilin fragment remains mainly in the pellet fraction (lane 4) of the recombinant 1637 HC coats that failed to uncoat: HC, clathrin heavy chain; 1675 HC, full-length recombinant clathrin heavy chain; 1637 HC, C-terminally truncated recombinant clathrin heavy chain; LCs, bovine brain light chains LCa1 and LCb2; rLCa1i, recombinant LCa1 expressed in insect cells; α/β, μ2, and ς2, components of the AP-2 complex; Auxilin, auxilin fragment (547-910).
Similar articles
- Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating.
Fotin A, Cheng Y, Grigorieff N, Walz T, Harrison SC, Kirchhausen T. Fotin A, et al. Nature. 2004 Dec 2;432(7017):649-53. doi: 10.1038/nature03078. Epub 2004 Oct 24. Nature. 2004. PMID: 15502813 - Structure of clathrin coat with bound Hsc70 and auxilin: mechanism of Hsc70-facilitated disassembly.
Xing Y, Böcking T, Wolf M, Grigorieff N, Kirchhausen T, Harrison SC. Xing Y, et al. EMBO J. 2010 Feb 3;29(3):655-65. doi: 10.1038/emboj.2009.383. Epub 2009 Dec 24. EMBO J. 2010. PMID: 20033059 Free PMC article. - Molecular and functional characterization of clathrin- and AP-2-binding determinants within a disordered domain of auxilin.
Scheele U, Alves J, Frank R, Duwel M, Kalthoff C, Ungewickell E. Scheele U, et al. J Biol Chem. 2003 Jul 11;278(28):25357-68. doi: 10.1074/jbc.M303738200. Epub 2003 May 5. J Biol Chem. 2003. PMID: 12732633 - Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis.
Eisenberg E, Greene LE. Eisenberg E, et al. Traffic. 2007 Jun;8(6):640-6. doi: 10.1111/j.1600-0854.2007.00568.x. Epub 2007 May 4. Traffic. 2007. PMID: 17488288 Review. - The role of molecular chaperones in clathrin mediated vesicular trafficking.
Sousa R, Lafer EM. Sousa R, et al. Front Mol Biosci. 2015 May 19;2:26. doi: 10.3389/fmolb.2015.00026. eCollection 2015. Front Mol Biosci. 2015. PMID: 26042225 Free PMC article. Review.
Cited by
- Combined nanometric and phylogenetic analysis of unique endocytic compartments in Giardia lamblia sheds light on the evolution of endocytosis in Metamonada.
Santos R, Ástvaldsson Á, Pipaliya SV, Zumthor JP, Dacks JB, Svärd S, Hehl AB, Faso C. Santos R, et al. BMC Biol. 2022 Sep 21;20(1):206. doi: 10.1186/s12915-022-01402-3. BMC Biol. 2022. PMID: 36127707 Free PMC article. - A sequential mechanism for clathrin cage disassembly by 70-kDa heat-shock cognate protein (Hsc70) and auxilin.
Rothnie A, Clarke AR, Kuzmic P, Cameron A, Smith CJ. Rothnie A, et al. Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):6927-32. doi: 10.1073/pnas.1018845108. Epub 2011 Apr 11. Proc Natl Acad Sci U S A. 2011. PMID: 21482805 Free PMC article. - Heat shock proteins in diabetes and wound healing.
Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, Roy S. Atalay M, et al. Curr Protein Pept Sci. 2009 Feb;10(1):85-95. doi: 10.2174/138920309787315202. Curr Protein Pept Sci. 2009. PMID: 19275675 Free PMC article. Review. - Reconstitution of Clathrin Coat Disassembly for Fluorescence Microscopy and Single-Molecule Analysis.
Böcking T, Upadhyayula S, Rapoport I, Capraro BR, Kirchhausen T. Böcking T, et al. Methods Mol Biol. 2018;1847:121-146. doi: 10.1007/978-1-4939-8719-1_10. Methods Mol Biol. 2018. PMID: 30129014 Free PMC article. - To the Surface and Back: Exo- and Endocytic Pathways in Trypanosoma brucei.
Link F, Borges AR, Jones NG, Engstler M. Link F, et al. Front Cell Dev Biol. 2021 Aug 6;9:720521. doi: 10.3389/fcell.2021.720521. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34422837 Free PMC article. Review.
References
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986;45:3–13. - PubMed
- Ehrlich M., Boll W., Van Oijen A., Hariharan R., Chandran K., Nibert M. L., Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. - PubMed
- Fotin A., Cheng Y., Grigorieff N., Walz T., Harrison S. C., Kirchhausen T. Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature. 2004a;432:649–653. - PubMed
- Fotin A., Cheng Y., Sliz P., Grigorieff N., Harrison S. C., Kirchhausen T., Walz T. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature. 2004b;432:573–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous