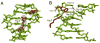Functional specificity of a Hox protein mediated by the recognition of minor groove structure - PubMed (original) (raw)
Functional specificity of a Hox protein mediated by the recognition of minor groove structure
Rohit Joshi et al. Cell. 2007.
Abstract
The recognition of specific DNA-binding sites by transcription factors is a critical yet poorly understood step in the control of gene expression. Members of the Hox family of transcription factors bind DNA by making nearly identical major groove contacts via the recognition helices of their homeodomains. In vivo specificity, however, often depends on extended and unstructured regions that link Hox homeodomains to a DNA-bound cofactor, Extradenticle (Exd). Using a combination of structure determination, computational analysis, and in vitro and in vivo assays, we show that Hox proteins recognize specific Hox-Exd binding sites via residues located in these extended regions that insert into the minor groove but only when presented with the correct DNA sequence. Our results suggest that these residues, which are conserved in a paralog-specific manner, confer specificity by recognizing a sequence-dependent DNA structure instead of directly reading a specific DNA sequence.
Figures
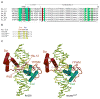
Figure 1. Overview of structures and sequences
A. Comparison of the YPWM, linker, and homeodomain sequences of a subset of Scr orthologs. Residue numbering is relative to the first homeodomain residue, which is +1. Residues highlighted in cyan distinguish Scr and its orthologs from other Hox paralogs. His-12 and Arg3 are highlighted in magenta. DNA contacting residues that are shared by all Hox paralogs are highlighted in green. Dm, Drosophila melanogaster; Ap, Apis melifera; Hs, Homo sapiens; Mm, Mus musculus; Dr, Danio rerio. B. Sequences of a generalized Hox-Exd site, and the fkh250con*, fkh250con, and fkh250 binding sites (in capital letters; identical flanking base pairs are shown in light grey). fkh250 and fkh250con*, which is a better match to the Hox-Exd consensus than fkh250con, were used for the crystallography. The base pairs in the Hox-Exd binding site are numbered 1 to 10. The Scr-Exd binding site in fkh250 is 100% conserved in all of the sequenced Drosophila genomes except for D. virilis, where only the last G has been changed to an A. C. Overview of the fkh250 (left) and fkh250con* (right) complexes. The Scr (red) and Exd (cyan) homeodomains bind to opposite faces of the DNA. Arg3 and His-12 are seen in the fkh250 complex but not in the fkh250con* complex. Base pairs colored in yellow are those that differ between fkh250 and fkh250con*.
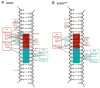
Figure 2. Protein-DNA contacts
Protein-DNA contacts for the (A) fkh250 and (B) fkh250con* complexes. The Exd half site is shaded cyan, the Hox half site is shaded red. Hydrogen bonds are represented by solid lines and non-polar interactions by dotted lines. Interactions involving the protein main chain are underlined. Green circles are visualized water molecules. The fkh250 complex has more water mediated contacts from residues such as Gln50, that is oriented differently in the two complexes, and Trp-17, that is positioned identically in the two complexes.
Figure 3. Minor groove insertion of Scr residues His-12 and Arg3 in fkh250
A. Electron densities for Arg3 and His-12 in the fkh250 complex, based on a simulated annealing Fo-Fc omit map (contoured at 3.0σ) B. Details of the His-12–Arg3 interaction and water-mediated interactions with Thy14, Thy29, and Thy30 of fkh250. The red circle marks a water molecule and dotted lines represent putative hydrogen bonds.
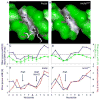
Figure 4. Differences in minor groove geometries and electrostatic potentials between fkh250 and fkh250con*
A,B: The surfaces of the (A) fkh250 and (B) fkh250con* DNAs, color-coded according to shape using GRASP (Nicholls et al., 1991). Black/gray and green surfaces represent concave and convex surfaces, respectively. Side chains for Arg5 (A and B) and Arg3/His-12 (A, only) entering the minor groove are shown in magenta. C,D: Graphs comparing minor groove widths seen in the crystal structures (blue curves) with those predicted by the MC simulations (green curves) for fkh250 (E) and fkh250con* (F). The sequences of the two binding sites are below; TpA steps are shown in bold lettering. E,F: Graphs comparing minor groove widths (Å) and electrostatic potential (kT/e) for fkh250 (E) and fkh250con* (F), based on the crystal structures. The positions of Arg5 and Arg3/His-12 minor groove insertion are indicated.
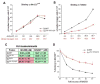
Figure 5. Binding of Scr and Scr mutants to fkh250 and fkh250con DNAs
A, B: Binding of ScrWT and ScrHis-12A,Arg3A to fkh250con (A) and fkh250 (B). Protein concentrations were chosen to give equivalent binding of ScrWT and ScrHis-12A,Arg3A to fkh250con (A), and the same concentrations were used to measure binding to fkh250 (B). All DNA binding measurements were done in the presence of HthHM-Exd (see Experimental Procedures). C: Binding affinities (Kds in nM) of the indicated proteins for fkh250 and fkh250con. Green and pink shaded boxes indicate interactions that can and cannot activate the respective reporter gene in vivo (data from Figure 6F-O). D: Competition experiment. Unlabeled fkh250 oligo at the relative concentrations indicated on the X axis was used to compete for the formation of ScrWT (black curve) and ScrHis-12A,Arg3A (red curve) complexes formed on a labeled fkh250con probe.
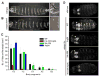
Figure 6. Compromised in vivo activities of Scr mutants
A: Ventral surface of wild type first instar larval cuticle. A′: higher magnification of wild type T1 segment showing a T1 beard (arrowhead). B: Ventral surface of a first instar cuticle after ubiquitous expression of ScrWT. B′: higher magnification of the T3 segment with an ectopic T1 beard (arrowhead). C: Quantification of ectopic T1 beard formation in thoracic (T2 and T3) and abdominal (A1 to A3) segments due to ubiquitous expression of ScrWT and Scr mutants. For comparison, the number of beard hairs in a wild type T1 segment is 128.2 +/-8.4. D: Ventral views of wild type embryos, or embryos ubiquitously expressing ScrWT or the indicated Scr mutant, stained for dCrebA. Ectopic activation is indicated by arrowheads.
Figure 7. ScrHis-12A,Arg3Ais unable to execute specific Scr functions in vivo
A-E: Wild type embryos or embryos ubiquitously expressing ScrWT or the indicated Scr mutants, stained for Fkh protein. Ectopic activation in anterior (arrowheads) and posterior (arrows) segments is indicated. Ventral views of PS1 to PS4 are shown. F-J: Expression of _fkh250_-lacZ, revealed by anti-β-gal antibody staining, in wild type embryos or embryos ubiquitously expressing ScrWT or Scr mutants. Ectopic activation in anterior (arrowheads) and posterior (arrows) segments is indicated. Shown are ventral views. K-O: Expression of _fkh250con_-lacZ, revealed by anti-β-gal antibody staining, in wild type embryos or embryos ubiquitously expressing ScrWT or Scr mutants. Ectopic activation in the head is indicated (arrowheads). Due to the already broad expression pattern in wild type embryos (K), lateral views are shown to visualize the ectopic expression.
Similar articles
- Deconvolving the recognition of DNA shape from sequence.
Abe N, Dror I, Yang L, Slattery M, Zhou T, Bussemaker HJ, Rohs R, Mann RS. Abe N, et al. Cell. 2015 Apr 9;161(2):307-18. doi: 10.1016/j.cell.2015.02.008. Epub 2015 Apr 2. Cell. 2015. PMID: 25843630 Free PMC article. - A unique Extradenticle recruitment mode in the Drosophila Hox protein Ultrabithorax.
Merabet S, Saadaoui M, Sambrani N, Hudry B, Pradel J, Affolter M, Graba Y. Merabet S, et al. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):16946-51. doi: 10.1073/pnas.0705832104. Epub 2007 Oct 17. Proc Natl Acad Sci U S A. 2007. PMID: 17942685 Free PMC article. - A structural model for a homeotic protein-extradenticle-DNA complex accounts for the choice of HOX protein in the heterodimer.
Chan SK, Mann RS. Chan SK, et al. Proc Natl Acad Sci U S A. 1996 May 28;93(11):5223-8. doi: 10.1073/pnas.93.11.5223. Proc Natl Acad Sci U S A. 1996. PMID: 8643557 Free PMC article. - Roles for intrinsic disorder and fuzziness in generating context-specific function in Ultrabithorax, a Hox transcription factor.
Bondos SE, Hsiao HC. Bondos SE, et al. Adv Exp Med Biol. 2012;725:86-105. doi: 10.1007/978-1-4614-0659-4_6. Adv Exp Med Biol. 2012. PMID: 22399320 Review. - The specificity of homeotic gene function.
Mann RS. Mann RS. Bioessays. 1995 Oct;17(10):855-63. doi: 10.1002/bies.950171007. Bioessays. 1995. PMID: 7487967 Review.
Cited by
- Role of Shape Deformation of DNA-Binding Sites in Regulating the Efficiency and Specificity in Their Recognition by DNA-Binding Proteins.
Sangeeta, Mishra SK, Bhattacherjee A. Sangeeta, et al. JACS Au. 2024 Jun 18;4(7):2640-2655. doi: 10.1021/jacsau.4c00393. eCollection 2024 Jul 22. JACS Au. 2024. PMID: 39055163 Free PMC article. - Deconvolving the recognition of DNA shape from sequence.
Abe N, Dror I, Yang L, Slattery M, Zhou T, Bussemaker HJ, Rohs R, Mann RS. Abe N, et al. Cell. 2015 Apr 9;161(2):307-18. doi: 10.1016/j.cell.2015.02.008. Epub 2015 Apr 2. Cell. 2015. PMID: 25843630 Free PMC article. - The N-terminus of the human RecQL4 helicase is a homeodomain-like DNA interaction motif.
Ohlenschläger O, Kuhnert A, Schneider A, Haumann S, Bellstedt P, Keller H, Saluz HP, Hortschansky P, Hänel F, Grosse F, Görlach M, Pospiech H. Ohlenschläger O, et al. Nucleic Acids Res. 2012 Sep 1;40(17):8309-24. doi: 10.1093/nar/gks591. Epub 2012 Jun 22. Nucleic Acids Res. 2012. PMID: 22730300 Free PMC article. - Predicting accurate ab initio DNA electron densities with equivariant neural networks.
Lee AJ, Rackers JA, Bricker WP. Lee AJ, et al. Biophys J. 2022 Oct 18;121(20):3883-3895. doi: 10.1016/j.bpj.2022.08.045. Epub 2022 Sep 3. Biophys J. 2022. PMID: 36057785 Free PMC article. - Structural and biophysical insights into the ligand-free Pitx2 homeodomain and a ring dermoid of the cornea inducing homeodomain mutant.
Doerdelmann T, Kojetin DJ, Baird-Titus JM, Solt LA, Burris TP, Rance M. Doerdelmann T, et al. Biochemistry. 2012 Jan 17;51(2):665-76. doi: 10.1021/bi201639x. Epub 2012 Jan 6. Biochemistry. 2012. PMID: 22224469 Free PMC article.
References
- Aggarwal AK, Rodgers DW, Drottar M, Ptashne M, Harrison SC. Recognition of a DNA operator by the repressor of phage 434: a view at high resolution. Science. 1988;242:899–907. - PubMed
- Billeter M, Qian YQ, Otting G, Muller M, Gehring W, Wuthrich K. Determination of the nuclear magnetic resonance solution structure of an Antennapedia homeodomain-DNA complex. J Mol Biol. 1993;234:1084–1093. - PubMed
- Burkhoff AM, Tullius TD. Structural details of an adenine tract that does not cause DNA to bend. Nature. 1988;331:455–457. - PubMed
- Casares F, Mann RS. Control of antennal versus leg development in Drosophila. Nature. 1998;392:723–726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM054510-15/GM/NIGMS NIH HHS/United States
- R01 GM054510/GM/NIGMS NIH HHS/United States
- U54 CA121852/CA/NCI NIH HHS/United States
- R01 AI041706/AI/NIAID NIH HHS/United States
- R01 GM054510-14/GM/NIGMS NIH HHS/United States
- R01 GM062947/GM/NIGMS NIH HHS/United States
- R24 GM074105-03/GM/NIGMS NIH HHS/United States
- R01 GM054510-13/GM/NIGMS NIH HHS/United States
- GM074105/GM/NIGMS NIH HHS/United States
- R01 GM054510-16/GM/NIGMS NIH HHS/United States
- GM54510/GM/NIGMS NIH HHS/United States
- R24 GM074105-04/GM/NIGMS NIH HHS/United States
- GM62947/GM/NIGMS NIH HHS/United States
- AI41706/AI/NIAID NIH HHS/United States
- R24 GM074105/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases